Article Text
Abstract
In the framework of the Quality Improvement Plan, a multidisciplinary working group on drug use improvement was created. The group was formed by three physicians from different specialities, two nurses, a pharmacist, a pharmacy technician and the coordinator of quality and patient safety. The work of the group was focused on improving the use of high risk medications in the hospital setting. The implementation of some of the improvements through the computerised physician order entry system was also considered. In the first 18 months of work, the group, in collaboration with other professionals and diverse departments of the hospital, implemented several improvements in drug safety: maxim dose alerts in electronic prescription, standardisation of dilution and dosages for intravenous high risk drugs, use of premixed solutions of potassium chloride, insulin protocols for electronic prescription in medical and surgical patients and emergency drug guidelines. The group has been extremely efficient in putting into practice protocols, procedures and guidelines for high risk medications with consensus and in a relatively short time.
This paper is freely available online under the BMJ Journals unlocked scheme, see http://ejhp.bmj.com/info/unlocked.dtl
Statistics from Altmetric.com
Introduction
In hospitals, to establish and apply drug safety measures and protocols is sometimes difficult and time consuming, some of the main problems being difficulty in organising meetings and arriving at a consensus between different professionals. In our hospital (200 beds, acute, non-profit with computerised physician order entry and nurse administration), in the framework of the Quality Improvement Plan, a multidisciplinary working group on drug use improvement was created. The main objectives of the group are to optimise and guarantee the proper use of medications, acting on the medical prescription, pharmaceutical validation, and dispensing and administration, thereby ensuring patient safety.
The group is composed of three physicians from different specialities (anaesthesia, internal medicine and surgery), two nurses, a pharmacist, a pharmacy technician and the coordinator of quality and patient safety. Due to the important safety issues on high alert medications,1 2 it was agreed within the group to focus on improving the use of these drugs in the hospital setting. Implementation of some of these measures through the computerised physician order entry system was also considered.3
Methods
The order of work was as follows. Firstly, after a bibliographic review, a list of drugs was established that was considered to be of high alert in our hospital. Secondly, strategies were discussed for the different drugs. Drafts of policies and protocols were elaborated within the group with the collaboration of other expert professionals in the hospital, as necessary (eg, the assistance of an endocrinologist was required for the elaboration of the insulin protocol). All procedures were sent for final assessment and approbation to the Pharmacy Committee. Finally, measures that could be computerised were implemented into the integrated system of clinical management (SAVAC-GFI) electronic health record system that includes all of the processes of drug therapy (physician order entry, pharmacist validation and nurse's record administration).
Results
After 18 months, the following improvements were proposed and carried out.
Maxim dose alerts
Maximum doses for antiarrhythmics, oral anticoagulants, dopamine and dobutamine were established. Automated alerts were displayed in the electronic prescription when doses higher than the limit were prescribed. Each time one of these drugs was prescribed at a dose higher than the maximum, the alert was displayed on the computer screen, enabling the physician to change the dose before confirmation/signature of the prescription. With these measures we tried to increase the barriers in the prescription process and guarantee security.
Standardisation of dilutions and dosages
Standardisation of dilutions and dosages, as well as electronic prescription protocols for high risk intravenous drugs in infusion pump administration, were established.
Firstly, protocols for standard doses and dilutions were agreed with physicians and nurses for intravenous mixtures of amiodarone, dopamine adrenergic and diuretic dosages, dobutamine, nitroglycerin, noradrenaline, labetalol, lidocaine, somatostanine and urapidil. Dilutions and dosages were standardised for both volumetric and syringe infusion pumps.
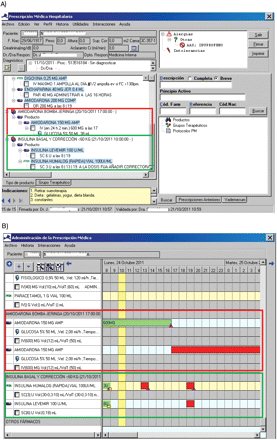
Secondly, to help physicians with the prescription, these protocols were loaded into the computerised physician order entry system. During the prescription process, when a protocol is chosen, the dose, diluent, concentration and rate of administration are loaded automatically, reducing the possibility of prescription and administration errors. The protocol is clearly visible in the electronic administration screen for the nurse, without any process of transcription (figure 1).
Examples of (A) e-prescription of amiodarone syringe infusion pump and insulin for a medical patient <60 kg of weight; and (B) nurse administration record for the same protocols (green indicates medication already administered).
A newsletter was published including all of the information on these protocols, and special sessions were organised with nursing staff because of their important role in preparation and administration. Double check techniques were also implied for these drugs.
Prescription of protocols showed a decrease in pharmaceutical interventions due to inaccurate prescribing of theses admixtures. In the 12 months before the implementation (April 2009–2010), the average numbers of pharmaceutical interventions were 40 per 100 prescriptions of these drugs. After implementation (April 2009–2010), the average has been reported to be 23%, with a descending trend as more physicians start using the protocols.
Insulin protocols
Insulin prescription was also proposed for improvement, as new treatments for diabetes that combine different insulins had been a source of problems (errors, doubts about dose interpretation by nurses, etc). Therefore, with the collaboration of the department of endocrinology, protocols for insulin dosage, based on patient weight, for medical and surgical patients were defined. These protocols were also automatically loaded into the prescription and could be directly visualised in a simple way by the nurse (figure 1).
Potassium chloride solutions
Even though on the wards concentrated solutions of potassium chloride (KCl) were stored in separate locked cupboard, another of the actions proposed by the group was the use of premixed solutions. In agreement with the different departments using this medication, it was decided which mixtures would be available in the hospital. Premixed solutions selected were included in the hospital formulary (eg, KCl 10 mEq in 500 ml of NaCl 0.9%; KCl 20 mEq in 500 ml of NaCl 0.9%; KCl 10 mEq in 500 ml of glucose 5%; KCl 20 mEq in 500 ml of glucose 5%; KCl 10 mEq in 500 ml of glucosalin; KCl 20 mEq in 500 ml of glucosalin). The wards and departments were they would be available were established, and they were separated from the other intravenous solutions and clearly identified. Information about the changes was provided through posters display in the wards as well as by direct communication. A protocol for electronic prescription of the premixed solutions was also devised. Concentrated solutions are now only available in the pharmacy service through prescription as restricted medications.
Before the introduction of the premixed solutions, the potential errors/problems detected during pharmaceutical validation of KCl prescriptions were 178 (April 2009–2010). This was reduced to 78 in the period April 2010–2011, all corresponding to concentrated KCl prescriptions in cases where there was no premixed solution suitable for the needs of the patient.
Emergency drugs guide
To improve access to information about emergency drugs, a guide has been published, adapted to the needs of the hospital, in a simple table format: drug, indication, dosage, recommendations and location of the different medications.4 The guide is available in all cardiac arrest carts of the hospital for fast and easy access.
Conclusions
The multidisciplinary composition of the group allowed us to approach and solve different issues concerning the safety of high alert medications in our hospital. Moreover, each of the components of the group has worked as a bridge to include other professionals in the preparation and implementation of the measures described.
Dissemination of the information on the improvements has led to greater awareness among professionals about high alert medications and the improvements carried out by the group. Very encouraging is the fact that in a relatively short time, several measures were agreed within different professionals and departments of the hospital.
Physicians found the prescription of these drugs very helpful and easy, opportunities for errors have been diminished and they feel that their diabetic patients are better controlled.
Key message
A multidisciplinary working group has led to fast and important improvements in drug safety.
The success of the group, who also work in collaboration with the Drug Error Committee and the Pharmacy Committee, has led to its continuation as a group with future work in progress, such as an antidote guide adapted to hospital needs and the preparation of guidelines for drug use in the elderly.
Acknowledgments
The authors acknowledge the assistance of Dr J Miralles (Pharmacy Director), Dr Biagetti (Department of Endocrinology) and the work of all of the members of the group: Dr Mahillo (Department of Anaesthesia), Dr Ruiz (Department of Internal Medicine), Dr García-Bonet (Department of Orthopaedic Surgery), C Borruell and S Quintela (nurses) and D Hernandez (pharmacy technician).
Footnotes
-
Competing interests None.
-
Provenance and peer review Not commissioned; externally peer reviewed.