Article Text
Abstract
Objectives An unacceptably high proportion of patients admitted to intensive care units (ICUs) develop drug-related problems (DRPs). DRPs might cause harm and increase costs and length of stay. The implementation of a clinical pharmacist service has been shown to detect a high number of DRPs and contributes effectively to solving these across different healthcare systems. However, this has not been prospectively studied in a mixed tertiary Norwegian ICU.
Methods During a 12-month period from October 2012, a clinical pharmacist was dedicated to review medications 3 h daily (Monday to Friday). DRPs were reported at the ICU conference and included advice by the pharmacist for each case. All DRPs were categorised and the clinical impact was documented for later analysis. Drug-related questions from the staff were categorised and answered.
Results 363 of 549 patients admitted to the ICU received medication reviews. 641 DRPs were detected in 194 of these patients (mean 1.8 DRPs per patient, range 0–25). Too high a dose, significant drug interactions and unnecessary or inappropriate drugs were among the most frequently detected DRPs. 87% of advice given by the pharmacist was accepted or taken into consideration. Typical questions from the nursing staff were related to drug preparation, generic equivalents and drug administration. Questions from doctors were most frequently related to drug dosage, efficiency and adverse effects.
Conclusions The addition of a dedicated clinical pharmacist to the ICU team improves the quality and safety of medication in a mixed Norwegian ICU.
- DRUG MANUFACTURING / PREPARATION / COMPOUNDING
- DOCUMENTATION AND ARCHIVES
- EDUCATION & TRAINING (see Medical Education & Training)
- DRUG PROCUREMENT
Statistics from Altmetric.com
- DRUG MANUFACTURING / PREPARATION / COMPOUNDING
- DOCUMENTATION AND ARCHIVES
- EDUCATION & TRAINING (see Medical Education & Training)
- DRUG PROCUREMENT
Introduction
Critically ill patients treated in the intensive care unit (ICU) suffer frequently from the risk of drug-related harm caused by adverse events and serious errors which might become life-threatening.1 Current illness and comorbidity can affect the pharmacokinetic and pharmacodynamic effects of drugs, such as distribution volume, protein binding and half-life. This makes dosing and the choice of drugs challenging. Moreover, patients who receive intensive care treatment are frequently prescribed several new drugs on top of their regular medication. Multiorgan failure, polypharmacy and a high proportion of intravenous drug use increase the risk of getting a drug-related problem (DRP). A DRP can be defined as an event or circumstance involving drug treatment that actually or potentially interferes with desired health outcomes.2
Clinical pharmacists are specifically trained in reviewing drug charts to identify DRPs and give advice to doctors on how to solve the problems. A study from Sweden demonstrated that the addition of pharmacists to healthcare teams led to major reductions in morbidity and healthcare costs.3 Other studies have shown a significant reduction in prescribing errors and drug-related patient harm (preventable adverse drug effects) by including a pharmacist in the ICU team.4–9 Implementation of a clinical pharmacist service has detected a high number of DRPs and contributed to solving DRPs in general wards and nursing homes in Norway.10 ,11 A small study in 23 Norwegian ICU patients has indicated that clinical pharmacists may increase the quality of drug treatment in a district hospital.12 However, this has not been prospectively confirmed on a larger scale.
We hypothesised that the addition of a dedicated clinical pharmacist would contribute significantly to improving the quality and safety of the medication processes in a mixed Norwegian ICU at a university hospital. We therefore aimed to study the contribution of clinical pharmacists to identifying and solving DRPs. We also aimed to monitor and classify questions from the staff and use them as indicators for the need for a clinical pharmacist support service in the ICU.
Methods
We applied a prospective design to evaluate the impact of having a dedicated clinical pharmacist in the ICU. The study was performed in a 10-bed mixed ICU at the University Hospital of North Norway from 15 October 2012 to 14 October 2013. This unit had not previously been staffed with dedicated clinical pharmacists. The pharmacists involved in the study (ETJ, SMH and ASM) had on average 2 years of experience as clinical pharmacists.
A pilot phase of 6 months was undertaken before the actual data collection period to develop and test different templates and methods which could be useful in the identification process of DRPs in the ICU. During the data sampling period one pharmacist attended the ICU from Monday to Friday between 09:00 and 12:00 h. All patients admitted to the ward between these hours were reviewed by the pharmacists, and there were no exclusion criteria except for weekends and holidays. All patients were followed up on a daily basis until discharged from the ICU.
The clinical pharmacist started every day with a brief review of the electronic hospital record focusing on the patient’s previous medical and drug history, current admission details, laboratory results and serum levels of drugs measured. Drug reconciliation was not systematically performed during the study period. However, the pharmacists spent a significant amount of time getting as much information as they could from various sources. A locally developed template was used to summarise the clinical context for each patient, which made it easier to follow up study patients. Information about current medication was collected from handwritten drug charts at the bedside and summarised in a data file.
From the collected information, the pharmacist reviewed current medication for the purpose of identifying potential DRPs. Interactions were identified through the following interaction databases (country of origin): http://www.interaksjoner.no (Norway), http://www.drugs.com (USA) and Stockley’s drug interactions: http://www.medicinescomplete.com/mc/alerts/current/drug-interactions.htm (UK). Choice of drug, dose, route and time of administration were compared with national and international guidelines and recommendations, combined with individual patient characteristics such as age, weight, clinical context, and renal and liver function. Examples of frequently used guidelines were: The Renal Drug Handbook,13 Handbook of Drugs in Intensive Care,14 http://www.legemiddelverket.no (Norwegian Summary of Products Characteristics), http://www.uptodate.com and http://www.relis.no (network of four regional medicines information and pharmacovigilance centres in Norway).
The patients’ need for their own regular medicines and possible adverse drug effects were also considered. The DRPs were classified using a validated method (table 1)15 and the drugs involved were recorded according to the Anatomical Therapeutic Chemical (ATC) classification system.
Classification of drug-related problems (DRPs)
Prior to this study, messages and advice from the pharmacists to the doctors were usually verbally handed over by the bedside nurse. This working pattern was radically changed at the initiation of the present study. The clinical pharmacist became a formal member of the already existing multidisciplinary team which met daily between 10:30 and 11:00 h (except holidays and weekends). In addition to a clinical pharmacist, ICU doctors, an infectious diseases consultant and the lead ICU nurse attended. During this meeting the pharmacist presented actual DRPs detected together with a pharmacy advice on how the DRPs could be solved. The pharmacy advice was subsequently discussed between the ICU doctors and the rest of the group and responses were recorded and classified by the pharmacist as accepted, rejected or to be followed up.
The actual changes to the patients’ drug charts after the counselling were also monitored by the pharmacists. These changes were categorised as: drug discontinued, addition of new drug, drug switch, dose reduction, dose enhancement, change of administration time and change of administration route/method.
A DRP was recorded as clinical relevant if pharmacy advice was directly accepted or taken into consideration by the clinical team. However, we did not perform any formal evaluation of the impact on clinical outcome. Data were collected from the clinical pharmacists’ notes and all DRPs reported in this study were detected by the clinical pharmacist only.
The clinical pharmacists were available for questions from ICU doctors and nurses on weekdays between 08:00 and 15:30 h. All questions were answered verbally or by email. The questions were categorised into the following groups: drug preparation, dosage, interactions, drug administration, identification, generics and pharmaceutical formulation, shelf life and storage, therapeutic drug monitoring, pharmacodynamic and pharmacokinetic properties, side effects and other questions.
In order to reach the entire nursing staff (90 nurses) and to properly answer all questions, the nursing staff were invited to formal lectures given by the pharmacist where frequently asked questions and issues in relation to DRPs were addressed.
Prior to this study, pharmacists designed customised drug information sheets for unlicensed drugs which formerly were only available as drug information leaflets written in non-English languages. The drug information sheets were made available for the staff through the hospital intranet as electronic pdf files. Based on DRPs recorded and questions asked by the clinical staff, pharmacists supplemented the drug sheets available during the study period.
In addition to the direct patient-centred clinical work, pharmacists were also responsible for providing and supplementing non-stock drugs prescribed by the ICU doctors.
Results
Five hundred and forty-nine patients were admitted to the ICU during the study period. Their mean (SD) age was 52 (22) years, length of stay in the ICU was 4.1 (5.7) days, time on mechanical ventilator was 2.9 (5.0) days, the Simplified Acute Physiology Score II was 41 (8), Nine Equivalents of Nursing Manpower Use Score was 183 (233) and ICU mortality was 15%.
Three hundred and sixty-three of the admitted patients were reviewed by a clinical pharmacist within the study period from Monday to Friday between 09:00 and 12:00 h. A total of 641 DRPs were detected in 194 patients, giving an average of 3.3 per patient (median 1, range 1–25). One hundred and sixty-nine patients did not have any DRP. The average DRP per patient for all patients including those without a DRP was 1.8. Figure 1 shows the distribution of DRPs per patient. There was no change in the numbers of DRPs during the study period.
Distribution of drug-related problems (DRPs) per patient in 194 patients during 1 year.
Figure 2 shows the distribution of DRPs across the different categories. Too high a dose accounted for 28% of the total number of DRPs detected, followed by significant drug interaction (16%) and drugs that were either unnecessary or contraindicated (13%).
Distribution of drug-related problems (DRPs) across the different categories in 194 patients during a 1-year period.
The drugs that were most frequently involved in the DRPs, categorised according to the ATC system, were antibacterials for systemic use (ATC-J01, 22%), antiepileptics (N03, 13%), drugs for acid-related disorders (A02, 9%), cardiac therapy (C01, 8%) and antithrombotic agents (B01, 8%).
The response of the ICU clinicians to the DRPs, divided into four categories, is shown in table 2. Figure 3 shows the clinical response of ICU consultants as a percentage across different DRP categories. Dose reduction was the single most frequently made change, followed by drug discontinued and new drug added to the chart. In addition, 43 blood samples were obtained on the request of the pharmacists in order to monitor drug levels.
Response of consultants to drug-related problems (DRPs) in 194 patients admitted to the intensive care unit (ICU)
Intensive care unit consultants’ clinical response to drug-related problems (DRPs) as a percentage across different DRP categories.
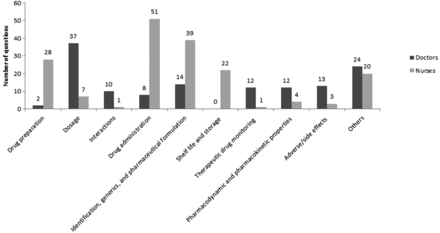
A total of 308 questions were received by the pharmacists, 176 from nurses and 132 from doctors. The questions were divided into categories as shown in figure 4. The nurses frequently asked about drug preparation, drug administration and identification and change of generic drugs. Furthermore, questions were often related to the administration of drugs via enteral feeding tubes or percutaneous endoscopic gastrostomy tubes. Other frequently asked questions were related to drug compatibility with other drugs and solutions administered via central venous catheters.
Questions divided into categories. A total of 308 questions were asked (176 by nurses and 132 by doctors).
The formal lectures provided to the nurses contained all the topics mentioned above. They also focused on how the nurses themselves could find answers to their own questions when pharmacists were unavailable. Frequently asked questions were often related to changes to the unit's drug formulary—for example, when digitoxin was replaced by digoxin and when fosphenytoin was replaced by phenytoin. These frequently asked questions and answers were also highlighted during the formal lectures.
Twenty-eight per cent of the questions from the doctors were related to drug dosage. Other issues brought up by the doctors were questions about drug efficiency and adverse drug effects.
The pharmacists received 70 questions from the nurses during the first 3 months, but this number was reduced over time with only 30 questions received during the last 3 months. A similar trend was recorded for the doctors with a reduction from 53 questions in the first 3 months to 32 questions during the last 3 months.
Discussion
The main finding of this study was that the implementation of a clinical pharmacist service improved the safety and quality of the entire medication process in a Norwegian mixed ICU.
DRPs are common in our unit, and a high proportion of them were clinically relevant as 87% of them were accepted or taken into consideration by the clinical team. A recently published study by Bourne et al,8 which is comparable to our study, showed an acceptance rate of 90%, while Lundereng et al12 reported an acceptance rate of 76%. Interpretation of the data from our study suggests that an ICU pharmacist service should include weekend and holidays to ensure that benefits of improved medication safety are offered to a higher proportion of patients rather than the 66% that were covered in this study. Our suggestion is also supported by several previously published studies.7 ,16 ,17
By bringing a multidisciplinary team together, we were able to improve the quality of the clinical decision processes. This was done through establishment of a formal quality assurance system for the entire medication process. The changes in working pattern for the ICU doctors were well received, and a relatively high acceptance rate for the DRPs (87%) indicates that ICU doctors acknowledge and accept the input from the clinical pharmacists. A positive attitude could also be due to the fact that the clinicians were invited to an ICU conference, which allowed them to discuss relevant clinical pharmacy issues on a daily basis. This might have reduced the burden on individual doctors and also increased the confidence in making correct clinical decisions in a complex multidisciplinary environment.
Differences in comorbidity, multipharmacy and length of stay (hours to about a month) can explain the large variation in the numbers of DRPs per patient. However, we believe this variation mirrors the everyday situation in most mixed ICUs. Patients who stay for a longer period are susceptible to getting more DRPs, but these patients were also better monitored. Increased attention over time from the attending clinical pharmacist might have increased the likelihood of detecting DRPs in this group of patients. Medication surveillance is particularly important in open ICUs where doctors rotate frequently and in units where other specialties are allowed to prescribe drugs. Implementation of a clinical pharmacy service in such units is more likely to reduce the number of DRPs detected and increase safety and quality.
We applied the validated method of Ruths et al15 to divide the DRPs into several categories. These categories were also similar to previously published studies from ICUs outside Norway5 ,8 but were not directly comparable with each other.
A large proportion of DRPs detected were due to drug interactions. However, it is important to remember that this particular study was performed in a hospital without any electronic medication charting system. The use of electronic interaction detecting systems might have reduced the total number of DRPs caused by drug interactions, but this remains to be studied in other healthcare systems comparable to ours. In any case, clinicians ought to take full responsibility for performing a complete review of the interactions of every drug prescribed to patients. Implementation of a clinical pharmacist service and/or access to electronic interaction detecting systems should not undermine the clinician’s responsibility in this respect.
The most frequent intervention in our study was dose reduction. During the daily multidisciplinary conference, dosage was frequently adjusted in accordance to the patient's age, comorbidity, body mass index and renal and liver function. Dose adjustments were also frequently made due to change in administration route.
As expected, several drugs were either discontinued or added to the drug chart during the admission. Drugs added to the drug chart were mostly regular drugs used at home by patients, such as eye drops against glaucoma and platelet inhibitors. Our observations underline the need for better control systems which harmonise the patient’s own medication list with the current in-hospital drug chart. These findings are also in line with data obtained from a small Norwegian district hospital.12
Transfer errors were another important reason why drugs were added to the chart. Patients admitted to the ICU are recruited mostly from the accident and emergency department and operating theatres but, in principle, they can be admitted from any department in the hospital. In order to reduce transcription errors, an electronic charting system is needed which could be used throughout the entire patient journey and without any restrictions on where the patients are actually receiving treatment. The regional healthcare authority has recently bought such a commercial available charting system, which will be implemented in the region. We would like to perform a follow-up study to determine the effects of implementing this specific electronic charting system in our health region.
Klopotowska et al5 found that the need for a change in dose and the addition of new drugs were the most frequently made interventions based on pharmacy advice. Bourne et al8 found that addition of a new drug, dose adjustment and administration optimisation were the most common changes in their study, which is in agreement with our findings. Medication errors at the administration stage are a serious safety problem in many ICUs.18 In our study, administration errors were difficult to review because the pharmacists did not systematically check whether nurses administered drugs as prescribed by the doctor. This is an important limitation of our study because administration of parenteral medication seems to represent a common pattern of weakness in patient safety in ICUs. Rothschild et al showed that failure to carry out the intended treatment correctly was the leading cause of medication errors.1 Based on the current data and our experience, we would recommend that pharmacists should spend more time in the ICU to investigate the incidence and reasons for administration errors.
Another weakness of our study is the limited time for reviewing possible adverse effects. This category is particularly difficult to review due to comorbidity and the fact that some patients received several new drugs while others were simultaneously discontinued. In more than 60% of cases, clinicians had to make a further consideration before any changes could be made (figure 3).
The doctor’s response to the DRPs detected was unknown in only 2% of cases. This was mainly due to poor communication or the fact that messages were not received or properly followed up by the next work shift. A fully digitalised charting system for the entire patient journey is highly desirable and is likely to increase the traceability of drugs prescribed. We also assume that an electronic charting system would improve and enhance the collaboration between ICU doctors and clinical pharmacists, but this assumption needs to be confirmed in future studies.
In addition to detecting and solving DRPs, pharmacists also received 308 questions from doctors and nurses during the study period. Most questions were received while the pharmacist was present in the ICU, which we believe reflects the importance of being physically present in the unit. The type of questions varied between the professions. A possible explanation for this difference could be the educational background, but it could also be due to the fact that nurses tended to ask questions at the bedside while doctors seemed to prepare and present their questions during the daily clinical conference meeting in front of a much bigger group. It therefore suggests that pharmacists should be available for both formal and informal meetings with the clinical staff, as the type of question could be context-dependent.
Moreover, by collecting and categorising all questions, we were able to prepare and deliver lectures that were customised to the nurses’ needs. This method is highly recommended for the purpose of spreading information to the ICU doctors as well.
We believe that formal lectures, easy access to drug information sheets in the user’s mother tongue and the daily staff meeting at 10:30 h were some of the reasons for the reduction in the number of questions during the pharmacist's ward rounds, and also contributed to safer drug management.
In conclusion, the addition of a dedicated clinical pharmacist to the ICU team improves the quality and safety of the entire medication process in a mixed Norwegian ICU.
Key messages
What is already known on this subject
An unacceptably high proportion of patients admitted to intensive care units (ICUs) outside Scandinavia develop drug-related problems (DRPs).
Implementation of a clinical pharmacist service in the ICU has been shown to solve DRPs across different healthcare systems.
However, clinical pharmacist services vary widely in Scandinavian ICUs and their impact has not been prospectively studied in a Norwegian ICU.
What this study adds
Implementation of a clinical pharmacist service confirms a high number of DRPs in a Norwegian mixed ICU.
87% of advice given by the pharmacist was clinically relevant as it was either accepted or taken into consideration by the ICU consultants.
Addition of a dedicated clinical pharmacist to the ICU team improves the quality and safety of the medication process in a mixed Norwegian ICU.
References
Supplementary materials
Bulgarian version
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Acknowledgements We would like to thank the doctors and nurses in the ICU who made this study possible.
Contributors All authors have contributed to the study design, data collection, interpretation and writing the final version of this manuscript.
Competing interests None declared.
Ethics approval This study was approved by the Institutional Regulatory Board at the University Hospital of North Norway.
Provenance and peer review Not commissioned; externally peer reviewed.