Article Text
Abstract
Objective To investigate whether a structured medication report at discharge from the hospital could reduce the number of medication discrepancies in primary care.
Method The study was performed as an open, randomised controlled study including patients transferred from one hospital in Norway to nursing home or home care. Both groups received epicrisis on discharge. In addition, the intervention group received a structured medication report. After discharge, the medication list in primary care service was compared with the list at discharge and medication discrepancies identified. In addition, these medication lists were retrospectively compared with the lists prior to admission to the hospital and at admission to hospital. A questionnaire on time spent and quality of the medication information was filled in by nurses in primary care.
Results Medication discrepancies were found for 72% (26) of the patients in the intervention group and 76% (42) in the control group (P=0.918). Most common was drugs omitted or committed to the medication lists in primary care service. Typically, the committed drugs in primary care were omitted drugs after admission to the hospital. Nurses used significantly less time (66%) obtaining medication information in the intervention group (P=0.041).
Conclusions Structured medication report as the only intervention did not reduce the medication discrepancies after discharge from hospital. There is a need for reconciliation at admission to ensure the quality of the medication report. Structured medication report resulted in the nurses spending less time on collecting medication information in primary care service.
- medication reconciliation
- medication discrepancies
- discharge from hospital
- primary care
- medication list
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Insufficient transfer of medication information is common when patients are transferred between healthcare levels.1–5 This is often attributed to poor communication between the healthcare levels and is known to increase the risk of medication errors.2 6 7 Patients with complex treatment including several medicines are transferred from hospital to primary healthcare and vice versa. This is an increasing challenge in the growing elderly population with comorbidity and polypharmacy.8
There are several systematic methods for ensuring correct medication information throughout the patient care pathway. One of the most documented is integrated medicine management (IMM) in which clinical pharmacists contribute to optimise the drug treatment for the individual patient.9–11 IMM is designed to cover all stages of in-patient care and includes the elements medication reconciliation at admission, medication review during the hospital stay and the use of structured medication reports documenting the changes in medication at discharge (figure 1).9 10 Medication reconciliation has been defined as ‘the process of identifying the most accurate list of a patient’s current medicines – including the name, dosage, frequency, and route- and comparing them to the current list in use, recognizing any discrepancies, and documenting any changes thus resulting in a complete list of medications, accurately communicated’.12 The effect of IMM has been well documented when all elements are included.9–11 13 However, implementation of the entire IMM model is time and resource demanding and is not easily achieved throughout the hospitals in Norway. In practice, therefore, only parts of the method are often implemented at the hospital wards. Structured medication report from the hospital is a tool to ensure the quality of the medication information transferred from hospital and has previously been shown to reduce the number of medication errors when patients are discharged from hospital.14
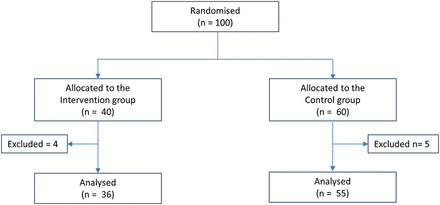
Inclusion of patients in the study.
Based on the practice of using only one element of IMM, the main objective of this study was to investigate whether introduction of a structured medication report at discharge from the hospital could reduce medication discrepancies (MDs). To avoid the challenges described by Eriksson et al,15 where implementation degree was influencing the results on medication error, we ensured that every patient in the intervention group received a structured medication report. Furthermore, we wanted to evaluate the effect structured medication reports had on the time nurses spent obtaining the medication list in primary care services.
Method
An open, randomised controlled study was performed to investigate whether a structured medication report from the hospital can reduce the number of MDs in the medication lists after discharge to primary care services: nursing homes or home care services. The primary end point of the study was the proportion of patients with at least one MD. MDs were defined as discrepancies in the medication list at primary care services within 10 days after discharge compared with the medication list at discharge from hospital. Changes documented by a general practitioner after discharge were not regarded as a discrepancy. The secondary end point of the study was the time primary care nurses spent on obtaining the medication information.
Patients from three medical wards at Akershus University Hospital, Norway, were included in the study during April 2013 to December 2014. The wards comprised 138 beds and were specialised in pulmonary disorders, neurology disorders or general internal medicine.
Patients were eligible for inclusion if they used medicines, were 18 years or older and received medical assistance from nursing homes or home care services in the districts of Lørenskog or Skedsmo. Patients competent to give consent and discharged from hospital were asked to participate in the study. The patients were included at the day of discharge from the hospital and were randomised between the control group and intervention group by use of coin flip. The patients gave their written informed consent before inclusion. Both groups received epicrisis that includes some medication information on discharge. In addition, the intervention group received a structured medication report. The structured medication report was based on the IMM template describing current, discontinued and new medicines, as well as any dose modifications. Changes in medication were justified in the medication report. The epicrisis was written by the prescribing hospital practitioner. The structured medication report was prepared by the study pharmacist based on the medical record, the medication prescribing system and the epicrisis, and was confirmed by the prescribing practitioner at the ward. The study pharmacist did not intervene with the hospital provider if a drug-related problem was found in the process. The epicrisis and the structured medication report were delivered to the patient at discharge from hospital. Information and teaching session were given by the study pharmacist regarding the project and the use of structured medication report at the relevant units prior to study start. Written information about the project was also distributed to the units upfront.
The patients in primary care had medication administration record as part of their care. Medication list was collected from primary care service within 10 days after discharge from hospital. The retrieved medication lists were then compared by the study pharmacist specialised in clinical pharmacy and trained in the IMM method. In this study, MDs were defined as discrepancies in the medication list in primary care compared with the list at discharge from hospital. The MDs were recorded and categorised into the following categories: commission of drugs (the patient did not use the drug at the hospital, but it was found in the medication list in primary care service), omission of drugs (the patient used the drug at the hospital, but it was not in the medication list in primary care service), incorrect strength of drug, incorrect drug formulation, incorrect dosing and incorrect administration time. The discrepancy was a MD when the change in medication list was not justified by a physician. A questionnaire prepared by the study group was administered to the relevant nurses for each patient in the primary care services. The questionnaire was tested in a small feasibility study including eight patients. The nurses registered time spent on obtaining the medical information, whether they found the information clear and comprehensible and which source they used to obtain the information. In addition, they rated the subjective experience on obtaining medication information on a scale from 1 (very bad) to 5 (very good). The completed questionnaires were collected by the study pharmacist within 10 days after discharge from the hospital.
For further evaluation, the following four medication lists for each patient were retrospectively compared: (1) in primary care service prior to admission, (2) at admission to hospital, (3) at discharge from hospital and (4) in primary care services within 10 days after discharge. The aim of this evaluation was to consider whether MDs found in primary care service after discharge could be explained by MDs introduced at admission to the hospital.
The sample size calculation was based on a study by Midlöv et al,14 where the introduction of a structured medication report reduced the proportion of patients with at least one discrepancy in their medication list from 66% in the control group to 32% in the intervention group. Assuming a similar level of discrepancies in this study and a possibility to achieve a similar reduction, with a 5% significance level and a power of 80%, 30 patients in each group were sufficient. To take into account the uncertainty when translating results from the previous study to the Norwegian context and the risk of withdrawals, we decided to include >30 patients in each group limited by the time period spent on inclusion of patients, for example, within 2014.
Statistical analyses were performed by use of the software Statistical Package for the Social Sciences V.21 , StataCorp 2015, Stata Statistical Software: Release 14 and Windows Excel 2013 (Microsoft Corporation, One Microsoft Way, Redmond, USA). CIs and P values were calculated by use of the statistical method bootstrap. To compare demographic variables, Fischer’s exact test was used to compare proportions, and Wilcoxon rank-sum test was applied to compare continuous variables.
Results
A total of 91 patients were included in the study (figure 1). Less than 20 patients declined to join the study. The main reason for exclusion during the study was misinformation at the hospital about the patient receiving support from home care nurse (six patients). One patient died, and the medication list for two patients could not be collected. Demographic data of the included patients are outlined in table 1.
Demographics of the study population
For the primary end point comparing medication list after discharge from hospital to primary care services, MDs were found in the medication lists for 72% (26) of the patients in the intervention group, on average 2.9 per patient. In the control group, MDs were found in 76% (42) of the medication lists, on average 3.0 per patient. There was no statistical significant difference between the groups, P=0.918.
The most common MD was commission of drugs to the medication list in the primary care services, as outlined in table 2.
Number of medication discrepancies (MD) with some examples
In addition to the primary end point where medication list at discharge from hospital was compared with the medication list in primary care services, these medication lists were also compared with the medication lists prior to admission.
Medicines for continuous use prescribed at the hospital were not found in the medication list in primary care services after discharge for 22% (8) of the patients in the intervention group and 22% (12) of the control group.
Medicines that were discontinued during hospital stay were reintroduced to 8% (three) of the patients in the intervention group and 4% (two) in the control group after transfer to primary care services.
The most common MD after the hospital stay when all four medication lists were compared was medicines reintroduced in primary care services although not administered at the hospital as outlined in table 3. Another typical MD was medicines for continuous use administered at the hospital not found in the medication list in primary care services, recorded for 55 MDs outlined as omission of drugs in table 2.
Most common medication discrepancies when medication lists were compared prior to admission, during hospital stay and after discharge from hospital
The questionnaires regarding time spent on obtaining the medical information, which source they used to obtain the information and their subjective experience on obtaining medication information were returned from 87 of the 91 nurses in the primary care services; 34 in the intervention group and 53 in the control group. The most common information source regarding the patients’ medicines was the written information from hospital, that is, epicrisis and/or the structured medication report. Only one (3%) nurse in the intervention and two (4%) in the control group reported contacting the hospital staff for information.
The nurses in the intervention group spent less time in obtaining medication information than the nurses in the control group (P=0.041), on average 11 and 25 min, respectively. In the intervention group, 88% (30) of the nurses rated the information as clear and comprehensible, while the corresponding result in the control group was 73% (38). Regarding the subjective experience with obtaining medication information, 92% (31) of the nurses in the intervention group rated good or very good (4 or 5) compared with 75% (40) in the control group.
Discussion
In this study, no statistical differences in MDs were seen with a structured medication report. Discrepancies were found for approximately 75% of the included patients. The most common MD when comparing all medication lists was omission of drugs at admission to hospital and commission of drugs after discharge, indicating that many of the discrepancies were due to lack of reconciliation at admission to the hospital. Justified hold in medication at the hospital was not recorded as MD. Nevertheless, the significant proportion of commission of medicine in primary care was actually the patient reintroduced to medicines they also should have received at the hospital. Indeed, the retrospective comparison of the medication lists indicated that the medication list in primary care after discharge mainly was restored to the one used prior to admission to the hospital. This is indicated by the small difference (eight MDs) in number regarding omission of drugs at admission to hospital and commission of medicines in primary care.
In some patients, reintroduction of medicines removed from the medication list at the hospital was seen, even when the removal was well-documented. Another typical MD was medicines for continuous use administered at the hospital not found in the medication list in primary care services outlined as omission of drugs in table 2. Although the introduction of these medicines was documented in the structured medication report, they were not included in the medicine list in primary care, indicating lack of confidence in the information from hospital. This corresponds to the results of other studies5 16 including a study by Glintborg et al.2 They found that one-fifth of medicines prior to admission were unknown to the hospital, and only half of the medications were listed in discharge summary. Similar results were also found in a previous Norwegian study.17
Structured medication report at discharge as a single intervention was not sufficient to reduce the number of MDs in this study. This is supported by the conclusions of two recent reviews on studies of care transition.7 18 These reviews conclude that there is conflicting evidence regarding efficacy of the interventions studied. However, interventions enforced with patient counselling and close collaboration between healthcare professionals were associated with improved efficacy.7 18
The results from our study are in contrast to previous studies on the IMM model focusing on the discharge summary14 19 which concluded that a medication report is an effective tool to reduce the number of medication errors. The differences compared with our study are that although a medication reconciliation was not performed in the study by Midlöv et al 14 the patient’s medication use was well known and documented prior to admittance to hospital. In the study by Bergkvist et al, 19 the quality of the medication report was improved by performing both medication reconciliation at admission and medication reviews throughout hospital stay, that is, the entire IMM method. Thus, as discussed above, the lack of medication reconciliation at admission to hospital in our study may be an explanation on the poor effect seen.
The nurses in primary care have the main responsibility on the practical work on updating the medication list by collecting information, and the time spent by the nurse was therefore chosen as the indicator regarding time spent. The physicians validate and approve the medication list. We have no evidence of miscommunication between the nurses and physicians in the primary care as this was not covered in a structured manner in this study.
However, the communication between the hospital and primary care services was poor seen by the fact that information given in the structured medication report or epicrisis about new prescriptions was not taken into account for >20% of the patients in the study population. Poor communication between different care levels when patients are transferred from hospital to municipal care has also been seen by others.2 4 6 7 20
The flip coin method used to randomise patients resulted in different group sizes. This could be seen as a limitation to the study. Nevertheless, the size of the intervention group was still adequate and the method ensured proper randomisation. Furthermore, the fact that this study was a randomised, controlled study should be considered an advantage compared with the previous studies.14 19 Since the structured medication report was written by the study pharmacist only and not implemented as a working procedure at the hospital wards, the risk of contamination between treatment groups is likely to be limited. The analysis of the main outcome measure was not blinded due to practical reasons, and assessment bias therefore cannot be excluded.
We had to distribute both the structured medication report and the epicrisis in the intervention group. Any effect of this, however, would contribute to reduce the observed difference between the groups in time spent, subjective satisfaction and assessment of comprehensiveness of the information, that is, the effect might be underestimated.
Conclusion
Structured medication report as the only intervention did not give any effect on the number of MDs in primary healthcare after discharge from hospital. One main reason for the lack of effect was the lack of reconciliation at admission that is necessary to ensure the quality of the medication report.
There is a need for an improvement in the communication between primary care and the hospital not only at discharge, but also at admission to the hospital.
Standardising the information in a structured medication report resulted in the nurses spending less time on obtaining information for the medication list than in the control group.
What this paper adds
What is already known on this subject
There is a need for an improvement in the communication between primary care and the hospital regarding medication when transferring patients.
The effect of medication management systems, like integrated medicine management (IMM), on reduction in medication discrepancies has been well documented when all elements are included.
However, implementation of the entire IMM model is time and resource demanding. In practice; therefore, only parts of the method are often implemented at the hospital wards in Norway.
What this study adds
Structured medication report alone was not sufficient to reduce the medication discrepancies after discharge from hospital. Reconciliation at admission is necessary to ensure the quality of the medication report.
There is a need for an improvement in the communication between primary care and the hospital not only at discharge, but also at admission to the hospital.
Standardising the information in a structured medication report saves time and effort for the nurses.
Acknowledgments
The authors thank the staff at the Neurology Department, the Pulmonary Department and the General Internal Medicine Department at Akershus University Hospital and the nursing homes and home care service in the districts of Lørenskog and Skedsmo. They are also grateful to the Health Services Research Centre at Akershus University Hospital for support in the statistical analyses.
References
Footnotes
EAHP Statement 4: Clinical Pharmacy Services.
Funding The authors are grateful for receiving funding from South-Eastern Norway Regional Health Authority (Helsedialog).
Competing interests None declared.
Ethics approval The Regional Committee for Medical and Health Research Ethics found no need for ethical approval of the study (document-id: 299683). The data protection officer at the hospital approved the study (12-081) in advance.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement The data are available for the authors and their institution.