Article Text
Abstract
Objectives In recent years, various formulations containing rapamycin, mainly petrolatum-based, have been tested on facial angiofibromas in tuberous sclerosis. They are often poorly tolerated due to irritation and bleeding. In addition, their effectiveness was insufficient in young adults. The objective of this study was to develop and characterise a hydro-alcoholic gel containing solubilised rapamycin. The stability of the product stored at 4°C was evaluated over 1 year.
Methods Two different 0.1% rapamycin gels were formulated with or without α-tocopherol and urea. Different methods were used to characterise the gels: HPLC, gas chromatography, pH, visual observation and optical microscopy. A physico-chemical and microbiological stability study was also conducted for 1 year at 4°C.
Results Gels were physically and microbiologically stable after 1 year at 4°C: organoleptic characteristics and pH unchanged, no significant decrease in rapamycin was observed, tocopherol droplet size was constant and rheological behaviour was not altered.
Conclusions This study describes a new gel formulation to improve skin penetration using various excipients to promote skin tolerance. This study provides, for the first time, detailed stability data for a hydro-alcoholic rapamycin gel.
- rapamycin
- topical formulation
- tuberous sclerosis
- stability
- angiofibromas
- hydrophilic gel
Statistics from Altmetric.com
Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant disease, characterised by the appearance of multiple hamartomas. Facial angiofibromas are one of the most common skin manifestations. They can be very disfiguring and can have a significant impact on patients' quality of life.1 They result from the overactivation of the mammalian target of rapamycin (mTOR) in dermal cells, which stimulates the proliferation of fibroblasts and keratinocytes and angiogenesis.2 3
Many symptomatic treatments have been developed to reduce the occurrence of angiofibromas (eg, cryosurgery, laser ablation…). But some of them are costly and the results are disappointing, with frequent recurrence and should require regular sessions.4 5 Recently, based on the pathophysiology of the disease, the interest of mTOR inhibitors such as rapamycin or everolimus has been studied. In order to limit systemic side effects due to oral administration, different topical forms have been prepared with these drugs. Rapamycin has been the most studied mTOR inhibitor in the past 10 years, used as an oral solution or as an ointment with crushed petrolatum tablets on the skin. Regardless of blood concentration, efficacy and safety have been demonstrated.6 7 However, Rapamune oral solution was not suitable for topical administration in terms of tolerance, causing irritation and burning sensations, probably due to high surfactant levels.8 Moreover, rapamycin being insoluble in petrolatum or Dexeryl, the cutaneous bioavailability of these preparations should be low, which explains a certain delay in reaching a clinical response and the lack of total disappearance in adults with severe angiofibromas. For children, a better response was observed probably due to their skin characteristics, i.e. thinner and more permeable.9 With regard to ointment with tablets crushed in petrolatum, the presence of solid fragments may be responsible for the risk of bleeding and an uncomfortable sensation of oily skin has been observed. Recently, Bouguéon et al proposed a cream with 0.1% rapamycin solubilised with 5% Transcutol to avoid any bleeding problem and this oily sensation, with stability data.10
Consequently, this work aimed to develop and characterise a hydro-alcoholic gel containing rapamycin solubilised and surfactant-free to improve the bioavailability of the active molecule, obtain a good appearance and allow skin tolerance. Finally, a long-term stability study at 4°C was performed to determine its shelf life.
Methods
Gel preparation
Two formulations were developed (table 1). Formula 1 (F1) was formulated without urea and α-tocopherol contrary to Formula 2 (F2). Carbopol-974P (Duchefa-Farma, Nederland) was used as a gelling agent. Rapamycin (Inresa, France) was solubilised in ethanol (VWR, France). Then, glycerol was added with or without α-tocopherol and urea at room temperature (Sigma-Aldrich, France). Twenty grams of gel were then packaged in aluminium tubes (Cooper, France) and stored in a controlled cold area (5°C±3°C) for the duration of the study.
Composition of rapamycin gel formulations
Chromatographic conditions
The method, based on the publication of Il’ichev et al,11 was validated according to the International Consensus on Harmonisation guidelines (ICHQ2R1). Samples were analysed using an Ultimate 3000 coupled with a Photodiode Array Detector PDA-3000 (Thermo-Fisher-Scientific, France) operating between 190 to 800 nm. Separation was achieved using an Interchim 250×4.6 mm KR-C18 (5 µm) column maintained at 40°C. The mobile phase A consisted of a mixture of water-formic acid 0.003 vol% (Merck, Germany) and mobile phase B was acetonitrile-formic acid 0.003 vol%. Acetonitrile and methanol (Chromasolv) were HPLC-grade (Sigma-Aldrich, France). The gradient programme used was an elution gradient of 50:50 to 10:90 (phase A:phase B) over 25 min. The flow rate was 1.0 mL/min and the injection volume was 50 µL. Rapamycin detection was processed at 277 nm.
Rapamycin extraction and recovery
To appreciate the extraction coefficient, five different rapamycin concentrations in gel were prepared (0.06, 0.08, 0.1, 0.12% and 0.14%) and measured by HPLC. For this, 0.1 g of the preparation was weighed and poured in a 10 mL volumetric flask. Methanol was added and the flask was placed in an ultrasonic bath for 10 min. The resulting solution was centrifuged in a Sigma centrifuge 1–14K at 10 000 rpm for 5 min and the supernatant was removed and analysed by HPLC. This solution was compared with rapamycin methanol stock solution at the same concentration.
Forced degradation
Stress studies were performed to demonstrate specificity of the stability-indicating method. To identify one major degradant of rapamycin, that is, secorapamycin, a standard solution at 10 µg/mL of secorapamycin (Cayman Chemical, USA) diluted in methanol, was subjected to the HPLC method.
For each formulation, 0.1 g of gel (n=3) was subjected to a stress condition of acid (0.1 n HCl: heated for 1 hour at 40°C), base (0.01 n NaOH: heated for 1 hour at 40°C), and oxidation (H2O2 1vol: heated at 40°C for 1, 2, 3, 6 and 24 hours, white light). Then, an extraction was performed under the same conditions as presented above and analysed by HPLC. Blank gel was also tested alone.
Organoleptic characteristics/pH
The different organoleptic characteristics evaluated were:
Appearance: An aliquot of gel was placed on a microscope slide and was inspected visually for their colour, homogeneity (presence of any aggregates) and grittiness (presence of particles or grits).
Consistency: The stickiness was evaluated by spreading an aliquot of blank gel (without rapamycin) on the skin.
Size and repartition of α-tocopherol droplets and detection of possible rapamycin crystals: Microscopic observation (OlympusIM coupled to a Sony camera XCD-U100CR) was performed to ensure the homogeneity of the gel by searching the absence of aggregates and following the evolution of the size of tocopherol droplets. This analysis was performed on about 100 units for each formula. The diameter, width and surface of lipid droplets were evaluated.
pH-determination: it was determined before each analysis using a calibrated pH-meter Consort-P901.
Determination of residual ethanol by gas chromatography
Residual ethanol was analysed using Ultra Trace GC gas chromatography system (Thermo Electron, Italy). The column was an Interchim 30 m x 0.53 mm UB-624 (3 µm) column maintained at 40°C for 10 min. The flow rate of carrier gas is 6 mL/min. A solution of acetonitrile 1 g/L (MeCN) was used as the internal standard. For the determination of residual ethanol, an aliquot of gel (50 mg) was weighed and poured with water and diluted in a 20 mL volumetric flask. Then, 1 µL of 50:50 (Sample:MeCN) was injected. The residual ethanol was expressed in percentage (g/100 mL).
Rheological measurements
Rheological measurements were performed on an Anton Paar MCR-102 (Modular Compact Rheometer, Austria) equipped with plate-plate geometry of 100 mm diameter and Peltier temperature control device with thermostatic hood. Viscosity was measured as a function of the shear rate for dγ/dt between 0.1 and 100 s-1 at 20.0°C±0.1°C.
Stability study
For the stability study, five batches were prepared for F1 (n=5) and four batches for F2 (n=4), and duplicate samples were analysed daily for each batch.
Samples (0.5 g) were withdrawn from tubes stored at 5°C±3°C, at different times (day): 0, 2, 7, 14, 30, 65, 100 and 365.
Different methods have been used to characterise both formulations, evaluate their microbial quality and study the drug physicochemical stability. For each time, the organoleptic characteristics and pH were evaluated, the percentage of rapamycin remaining and the residual ethanol concentration. The rheological properties were evaluated at day 0, 30 and 365, and the microbiological cleanliness was evaluated at day 0 and day 365.
The expiry date (days), based on a limit of 5% degradation, was calculated from the determination of T95% as part of the process of assigning product shelf life in accordance with the recommendations of ICH.
Microbiological stability
Microbial contamination of formulations has been tested in accordance with the European Pharmacopoeia. 1 g sample to be tested, freshly manufactured or already stored for 1 year at 4°C were diluted to 1/50 in meat peptone neutral solution. 5 mL of solution was filtered at a rate of 1 filter/ml. Thereafter, the filters were successively rinsed with 50 mL sodium chloride solution and with 50 mL meat peptone neutral solution to remove residual ethanol.
After contact of a filter membrane with soybean casein digest agar, the plates were incubated at 32°C and total aerobic microbial count (TAMC) was determined after 3-day storage. The use of Sabouraud dextrose agar incubated at 25°C for 5 days determined the total combined yeasts/moulds count (TYMC). Results were expressed as a colony forming unit per gram (CFU/g) against specification limits (<10² CFU/g for TAMC and<10 CFU/g for TYMC).
Statistical analysis
All values were expressed as mean±SD deviation. Data were compared using one-way ANOVA. In all cases, a difference was considered significant at P<0.05.
Results
Characterisation of the formulations
Physical and chemical characteristics of rapamycin gels
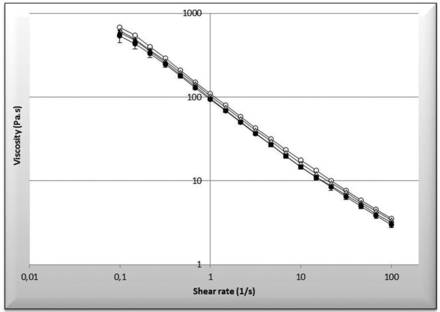
The organoleptic characteristics and pH of the different gels are summarised in table 2. Both formulations were smooth, homogeneous with a slight alcohol odour and a pH was compatible with skin application. A slight opalescence was observed in F2 due to tocopherol droplets. In addition, microscopic observations revealed the absence of rapamycin crystals in both formulations, confirming the dissolution of the drug in these gels. Both formulations had a rheofluidifying character. The viscosity of the gels is close to 20 Pa.s, completely compatible with a cutaneous application.
Organoleptic characteristics and pH values for each gel formulation
Determination of residual ethanol by gas chromatography
The GC chromatogram showed that ethanol and acetonitrile were eluted at 2.4 min and 2.9 min, respectively. The ethanol concentrations were 18.5%±1.5% for F1 and 18.7%±2.8% for F2, respectively.
Rapamycin content in gels
Validation of the HPLC method to measure rapamycin content in gel
This method permitted the obtainment of a well-defined main peak (asymmetry factor=1) and a good resolution to ensure a good separation between isomers. As shown in figure 1, the retention times were 20 min and 21 min for the rapamycin beta (β) and gamma (γ) isomer, respectively. Linear response signal versus concentration was obtained (r2=0.998). The mean recovery rate was 100.03%±1.3%. The relative SD were 1.3% and 2.0% for the repeatability and the intermediate precision, respectively.
Typical overlay RP-HPLC chromatograms of blank gel (black line), rapamycin gel (blue line) isomers β (1) and γ (2) after methanol extraction. Standard solution at 10 µg/mL of secorapamycin in methanol (pink line). Column: 5 µm, Interchim C18 250×4.6 mm; temperature 40°C; injection volume 50.0 µL; and UV detection 277 nm. Mobile phase: water/formic acid 0.003 vol% and acetonitrile/formic acid 0.003 vol%.
Rapamycin extraction and recovery
The extraction in methanol from the gel, allowed a recovery of 92.2%±0.1% for F1 and 94.6%±0.1% for F2. The drug content in gels was 0.098%±0.003% for F1 and 0.090%±0.005% for F2.
Forced degradation
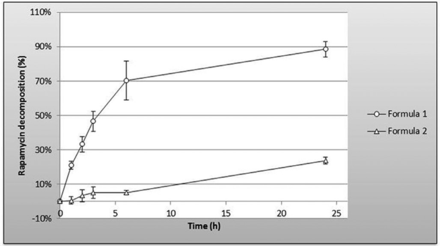
No interfering peak was observed regardless of the stressed condition applied. Adequate resolution between the peaks relevant to rapamycin and rapamycin degradation products was also observed. Moreover, the addition of tocopherol in F2 protects rapamycin against oxidative stress: %rapamycin decomposition at 24 hours was 88.6%±4.4% versus 23.6%±2.0% for F1 and F2, respectively (figure 2).
Oxidative stress of gel formations analysed by RP-HPLC. Stressed conditions: H2O21 vol; heated at 40°C for 1, 2, 3, 6 and 24 hours, white light. The addition of tocopherol in Formula 2protects rapamycin from oxidative stress.
Stability study
Physicochemical characteristics
The organoleptic characteristics (appearance, modification of colour, stickiness) of the gels remained unchanged throughout the period tested. No particle of rapamycin was detected by microscopic examination. Moreover, no modification of rheological character of gels was observed as a function of time (figure 3).
Rheological measurement over time of the gel. Empty round corresponds to Formula 1 at day 0 versus full round corresponds to Formula 1 at 360 days. Empty triangle corresponds to Formula 2 at day 0 versus full triangle corresponds to Formula 2 at 360 days.
The α-tocopherol droplets size did not significantly change throughout the storage period (8.2±5.3 µm, P=0.09).
Regarding pH, no significant change after 1 year of storage was observed, pH were 6.7±0.1 (P=0.13) for F1 (n=3) and 6.7±0.08 (P=0.71) for F2 (n=3).
Determination of residual ethanol by gas chromatography
Throughout the storage period, residual ethanol did not significantly alter (P=0.92 and P=0.40 for F1 and F2, respectively). The solvent was within 20.0%±2.2% for F1 (n=5) and 20.5%±2.5% for F2 (n=4) as originally found. From the beginning of the study, a 33% loss was observed with respect to the theoretical concentration (ie, 30% weight/weight).
Percentage of rapamycin remaining
The evolution of the remaining percentage of rapamycin is indicated in table 3. No significant change occurred after 1 year storage (P=0.17 for F1 and P=0.12 for F2). The mean concentration levels were 99.4%±2.2% and 101.0%±1.9% for F1 (n=5) and F2 (n=4), respectively. Due to these results, the T95% was not calculated because there was no evidence that the gels can be stored more than 1 year without any loss of drug. As shown in figure 4, both chromatograms at days 0 and 365 for F2 were comparable. The same result was observed with F1. Moreover, the degradation products, notably secorapamycin, were undetected.
Remaining percentage of rapamycin in preparations over the time
Chromatogram of rapamycin gel at D0 versus D365 (Formula 2). Both peaks were surimposable, which reflected the stability of the product over the time in the preparation. The same result was observed with Formula 1.
Microbiological stability
All preparations conformed to the current European Pharmacopoeia 8.0 and showed that the TAMC and the TYMC were less than 10 UFC/g in the storage conditions at 4°C. In addition, there were no suspected colonies of Staphylococcus aureus and Pseudomonas aeruginosa found in the selective media of any samples. Moreover, the same result was observed after 1 year of storage.
Discussion
The results showed that the hydro-alcoholic gel containing 0.1% rapamycin and formulated with or without α-tocopherol and urea, was stable for at least 1 year at +4°C and protected from light. No degradation products were detected and there were no changes in the physico-chemical or microbiological characteristics of the gel. The gel had a smooth, homogeneous texture with no trace of rapamycin precipitate. In addition, the residual ethanol content remained stable for 1 year. Nevertheless, a loss of 33% ethanol after manufacture compared with the theoretical concentration was observed. This loss is probably due to the manufacturing process since the stability study revealed a constant percentage. This loss could have resulted in rapamycin precipitation, but microscopic analysis of the gel did not detect undissolved rapamycin particles. In F2, the droplet size distribution of α-tocopherol remained unchanged after 1 year, which implies good physical stability.
Among the different types of topical preparations based on rapamycin, a single cream containing 0.1% rapamycin solubilised in Transcutol and mixed with a marketed cream (Excipial) was studied for its stability.10 However, stability has only been achieved over 85 days while our gel is stable at 4°C for a shelf life of at least 1 year. Moreover, our results were obtained using a highly selective and validated RP-HPLC method, allowing good separation of isomers and degradation products, including secorapamycin, an open ring isomer with extremely low immunosuppressive activity.11 In addition, it has been shown that this method indicates stability with a very good extraction coefficient (more than 90% for both formulas in hydro-alcoholic gel compared with 64.2%±1.2% in Excipial).10
The choice to develop a 0.1% rapamycin hydrogel was based on the literature. As shown by several studies, the efficacy and tolerance of the different preparations used are more or less good, depending on the concentration of rapamycin, the type of raw material (API standard powders, ground tablets or oral solutions of rapamycin), the vehicle used for the topical route and the age of the patients.6 8 9 12 13
It should be noted that the use of crushed tablets or rapamycin powder, dispersed in a fatty base such as petrolatum or Dexeryl, would limit skin bioavailability because the drug is not fully solubilised in the topical formulation. Therefore, most authors used concentrations up to 0.1%, particularly 0.2% and 1%, or increased the frequency of application (twice daily).6 7 14 15 In a prospective study, Malissen et al showed a significant improvement in FASI by using 1% Dexeryl rapamycin cream, resulting in complete clearance of 50% at 9 months, with no further benefit.15 In addition, it was assumed that the bleeding was due to incomplete crushing of the tablets. The oral solution was also very irritating, probably due to the high level of surfactant, and made the skin oily, leading to poor compliance.12 16
So, in order to limit tolerance problems and to increase skin bioavailability, rapamycin solubilisation has been identified as a key element of the process. Ethanol was chosen as the solubilising solvent. It is well known that ethanol is capable of improving the cutaneous penetration of drugs by increasing the porosity of the stratum corneum.17 To increase patient comfort, we have added some excipients to our formulation. Glycerol is a hygroscopic substance used as a humectant and emollient agent to promote skin hydration.18 In addition, topical application of glycerol improves skin elasticity and helps repair the epidermal barrier.19 The addition of α-tocopherol, an effective antioxidant, has been used to protect rapamycin from oxidation and also to protect the very damaged skin of these patients, thanks to an anti-radical action.20 Urea is traditionally used in dermatology for its moisturising and keratolytic action,21 which can be beneficial for patients with dry skin and hyperkeratosis. However, even if the low concentration used in gels is not sufficient to allow a keratolytic effect, urea can still act as an emollient. Our formulation meets both the objective of treating angiofibroma, as all studies published to date, and improves skin tolerance by adding the excipients mentioned above.
Finally, carbopol was added to our formulation because of its excellent tolerance and drug delivery capability. In addition, carbopol gels containing penetration activators such as ethanol have proven to be very effective and relatively easy to prepare.
No antimicrobial agents have been added due to the presence of ethanol and the bacteriostatic and antifungal properties of rapamycin.22
The gel formulation offers better application characteristics than cream and ointment. Moreover, as Tanaka et al have demonstrated, the gel allows greater skin penetration than ointment according to in vitro tests and low irritation.23 Finally, the hydrophilic character induces a better aesthetic comfort for the patient and a better compliance.
In a recent study, Wataya-Kaneda et al concluded that the optimal concentration of rapamycin hydrophilic gel was 0.2%, but observed more adverse events in the 0.2% rapamycin subgroup with dry skin (in 62% of cases) and irritation (in 50% of cases). In addition, low blood levels of rapamycin were detected in several patients, particularly in the paediatric subgroup (50% and 100% in the adult subgroup and paediatric subgroup respectively), resulting in the likely occurrence of systemic side effects.24 In this context, our study proposed that 0.1% should appear as the highest concentration of rapamycin to achieve an excellent therapeutic index without any systemic side effects for the patient and at a lower cost.
Finally, the long stability obtained with our formulation, allows optimal patient management given the frequency of consultations (every 6 months minimum) and the frequent use of treatment beyond this consultation period.
Conclusion
This study describes a new gel formulation that is stable for 1 year, which could be effective against angiofibromas and safe in adulthood. Rapamycin has been solubilised in ethanol to improve skin penetration and various excipients have been added to promote tolerance. In addition, the hydrophilic nature of the formulation induces aesthetic comfort for the patient.
What this paper adds
What is already known on this subject
Topical rapamycin is an effective and safe medical alternative to ablative treatments in patients with TSC-associated facial angiofibromas
No curative treatment has been marketed in Europe
Only one study published stability data on a cream but stability has only been achieved over 85 days
What this study adds
This work would be the first to have studied a long-term physico-chemical stability of a new hydrophilic rapamycin gel with detailed data (1 year)
This is the first study to propose the use of various excipients in order to improve skin penetration and skin tolerance
Acknowledgments
The authors acknowledge Vincent Boudy and Benoit d’Hayer for their help during the rheological measurements and Bernard Do for his language assistance.
References
Footnotes
EAHP Statement 3: Production and Compounding.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent Not required.
Provenance and peer review Not commissioned; externally peer reviewed.