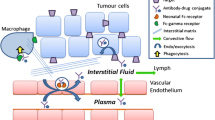
Abstract
Antibody–drug conjugates (ADCs) are developed with the goal of increasing compound therapeutic index by specific and targeted delivery of a toxic payload to the site of action while considerably reducing damage to normal tissues. Yet, off-target hepatic toxicities have been reported for several ADC. Locations of these off-target toxicities coincide with the reported locations of cell surface mannose receptor (MR). The relative proportion of agalactosylated glycans on the Fc domain (G0F vs. G1F and G2F components) in monoclonal antibody (mAb)–based biotherapeutics is closer to some disease state IgG rather than to a normal serum-derived immunoglobulin. The lack of the terminal galactose on a G0F glycan creates an opportunity for the mAb to interact with soluble and cell surface MRs. MR is a known multi-domain lectin that specifically binds and internalizes glycoproteins and immune complexes with relatively high G0F content and has been found on the surface of various cell types, including immune cells of myeloid lineage, endothelial cells, and hepatic and splenic sinusoids. In this review paper it is proposed that the mechanism of the off-target toxicities for ADC biotherapeutics is at least in part driven by the carbohydrates, specifically agalactosylated glycans, such as G0F, their interactions with MR and resulting glycan-derived cellular uptake of ADCs. Several case studies are reviewed presenting corroborating information.
Similar content being viewed by others
References
Adamo M, Qiu D, Dick LW Jr, Zeng M, Lee A-H, Cheng K-C (2009) Evaluation of oligosaccharide methods for carbohydrate analysis in a fully human monoclonal antibody and comparison of the results to the monosaccharide composition determination by a novel calculation. J Pharm Biomed Anal 49:181–192
Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P (2003) Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol 325:979–989
Allavena P, Chieppa M, Monti P, Piemonti L, Allavena P, Chieppa M, Monti P, Piemonti L (2004) From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor. Crit Rev Immunol 24:179–192
Jones AJS, Papac DI, Chin EH, Keck R, Baughman SA, Lin YS, Kneer J, Battersby JE, Jones AJS, Papac DI, Chin EH, Keck R, Baughman SA, Lin YS, Kneer J, Battersby JE (2007) Selective clearance of glycoforms of a complex glycoprotein pharmaceutical caused by terminal N-acetylglucosamine is similar in humans and cynomolgus monkeys. Glycobiology 17:529–540
Linehan SA, Linehan SA (2005) The mannose receptor is expressed by subsets of APC in non-lymphoid organs. BMC Immunol 6:4
Onda M, Kreitman RJ, Vasmatzis G, Lee B, Pastan I (1999) Reduction of the nonspecific animal toxicity of anti-Tac(Fv)-PE38 by mutations in the framework regions of the Fv which lower the isoelectric point. J Immunol 163:6072–6077
Battelli MG, Buonamici L, Polito L, Bolognesi A, Stirpe F (1996) Hepatoxicity of ricin, saporin or a saporin immunotoxin: xanthine oxidase activity in rat liver and blood serum. Virchows Arch 427:529–535
Tolcher AW, Ochoa L, Hammond LA, Patnaik A, Edwards T, Takimoto C, Smith L, de Bono J, Schwartz G, Mays T, Jonak ZL, Johnson R, DeWitte M, Martino H, Audette C, Maes K, Chari RV, Lambert JM, Rowinsky EK (2003) Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg antigen: a phase I, pharmacokinetic, and biologic correlative study. J Clin Oncol 21:211–222
Mosure KW, Henderson AJ, Klunk LJ, Knipe JO (1997) Disposition of conjugate-bound and free doxorubicin in tumor-bearing mice following administration of a BR96-doxorubicin immunoconjugate (BMS 182248). Cancer Chemother Pharmacol 40:251–258
Onda M, Willingham M, Wang QC, Kreitman RJ, Tsutsumi Y, Nagata S, Pastan I (2000) Inhibition of TNF-alpha produced by Kupffer cells protects against the nonspecific liver toxicity of immunotoxin anti-Tac(Fv)-PE38, LMB-2. J Immunol 165:7150–7156
Giles FJ, Kantarjian HM, Kornblau SM, Thomas DA, Garcia-Manero G, Waddelow TA, David CL, Phan AT, Colburn DE, Rashid A, Estey EH (2001) Mylotarg (gemtuzumab ozogamicin) therapy is associated with hepatic venoocclusive disease in patients who have not received stem cell transplantation. Cancer 92:406–413
Gillespie AM, Broadhead TJ, Chan SY, Owen J, Farnsworth AP, Sopwith M, Coleman RE (2000) Phase I open study of the effects of ascending doses of the cytotoxic immunoconjugate CMB-401 (hCTMO1-calicheamicin) in patients with epithelial ovarian cancer. Ann Oncol 11:735–741
Kamoda S, Ishikawa R, Kakehi K (2006) Capillary electrophoresis with laser-induced fluorescence detection for detailed studies on N-linked oligosaccharide profile of therapeutic recombinant monoclonal antibodies. J Chromatogr A 1133:332–339
Bailey MJ, Hooker AD, Adams CS, Zhang S, James DC (2005) A platform for high-throughput molecular characterization of recombinant monoclonal antibodies. J Chromatogr B 826:177–187
Weitzhandler M, Hardy M, Co MS, Avdalovic N (1994) Analysis of carbohydrates on IgG preparations. J Pharm Sci 83:1670–1675
Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA (2007) The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol 25:21–50
Routier FH, Hounsell EF, Rudd PM, Takahashi N, Bond A, Hay FC, Alavi A, Axford JS, Jefferis R (1998) Quantitation of the oligosaccharides of human serum IgG from patients with rheumatoid arthritis: a critical evaluation of different methods. J Immunol Methods 213:113–130
Arnold JN, Dwek RA, Rudd PM, Sim RB (2006) Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunol Lett 106:103–110
PMDA (2007) Bevacizumab (Avastin). Report on deliberation results, March 6, 2007. Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare
Kamoda S, Nomura C, Kinoshita M, Nishiura S, Ishikawa R, Kakehi K, Kawasaki N, Hayakawa T (2004) Profiling analysis of oligosaccharides in antibody pharmaceuticals by capillary electrophoresis. J Chromatogr A 1050:211–216
Chen X, Liu YD, Flynn GC (2009) The effect of Fc glycan forms on human IgG2 antibody clearance in humans. Glycobiology 19:240–249
Higuchi Y, Nishikawa M, Kawakami S, Yamashita F, Hashida M (2004) Uptake characteristics of mannosylated and fucosylated bovine serum albumin in primary cultured rat sinusoidal endothelial cells and Kupffer cells. Int J Pharm 287:147–154
Dong X, Storkus WJ, Salter RD (1999) Binding and uptake of agalactosyl IgG by mannose receptor on macrophages and dendritic cells. J Immunol 163:5427–5434
Kogelberg H, Tolner B, Sharma SK, Lowdell MW, Qureshi U, Robson M, Hillyer T, Pedley RB, Vervecken W, Contreras R, Begent RH, Chester KA, Kogelberg H, Tolner B, Sharma SK, Lowdell MW, Qureshi U, Robson M, Hillyer T, Pedley RB, Vervecken W, Contreras R, Begent RHJ, Chester KA (2007) Clearance mechanism of a mannosylated antibody-enzyme fusion protein used in experimental cancer therapy [erratum appears in Glycobiology. 2007 Oct; 17(10):1030]. Glycobiology 17:36–45
Biessen EA, van Teijlingen M, Vietsch H, Barrett-Bergshoeff MM, Bijsterbosch MK, Rijken DC, van Berkel TJ, Kuiper J (1997) Antagonists of the mannose receptor and the LDL receptor-related protein dramatically delay the clearance of tissue plasminogen activator. Circulation 95:46–52
Bijsterbosch MK, Donker W, van de Bilt H, van Weely S, van Berkel TJ, Aerts JM (1996) Quantitative analysis of the targeting of mannose-terminal glucocerebrosidase. Predominant uptake by liver endothelial cells. Eur J Biochem 237:344–349
Lee RT, Wang M-H, Lin W-J, Lee YC (2011) New and more efficient multivalent glyco-ligands for asialoglycoprotein receptor of mammalian hepatocytes. Bioorg Med Chem 19:2494–2500
Wu AM, Senter PD (2005) Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol 23:1137–1146
Mylotarg® (2007) Wyeth Pharmaceuticals Inc., Philadelphia, PA
Kim YW, Chang TW (1992) Potential use of immunoconjugates for AIDS therapy. AIDS Res Hum Retroviruses 8:1033–1038
Byers VS, Rodvien R, Grant K, Durrant LG, Hudson KH, Baldwin RW, Scannon PJ (1989) Phase I study of monoclonal antibody-ricin A chain immunotoxin XomaZyme-791 in patients with metastatic colon cancer. Cancer Res 49:6153–6160
Weiner LM, O’Dwyer J, Kitson J, Comis RL, Frankel AE, Bauer RJ, Konrad MS, Groves ES (1989) Phase I evaluation of an anti-breast carcinoma monoclonal antibody 260F9-recombinant ricin A chain immunoconjugate. Cancer Res 49:4062–4067
Skilleter DN, Price RJ, Parnell GD, Cumber AJ (1989) The low uptake of an abrin A-chain immunotoxin by rat hepatic cells in vivo and in vitro. Cancer Lett 46:161–166
Skilleter DN, Foxwell BM (1986) Selective uptake of ricin A-chain by hepatic non-parenchymal cells in vitro. Importance of mannose oligosaccharides in the toxin. FEBS Lett 196:344–348
McKoy JM, Angelotta C, Bennett CL, Tallman MS, Wadleigh M, Evens AM, Kuzel TM, Trifilio SM, Raisch DW, Kell J, DeAngelo DJ, Giles FJ, McKoy JM, Angelotta C, Bennett CL, Tallman MS, Wadleigh M, Evens AM, Kuzel TM, Trifilio SM, Raisch DW, Kell J, DeAngelo DJ, Giles FJ (2007) Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): an overview from the research on adverse drug events and reports (RADAR) project. Leuk Res 31:599–604
Rajvanshi P, Shulman HM, Sievers EL, McDonald GB, Rajvanshi P, Shulman HM, Sievers EL, McDonald GB (2002) Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy [erratum appears in Blood 2002 Jun 1; 99(11):3915]. Blood 99:2310–2314
Goetze AM, Liu YD, Zhang Z, Shah B, Lee E, Bondarenko PV, Flynn GC (2011) High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 21:949–959
Alessandri L, Ouellette D, Acquah A, Rieser M, Leblond D, Saltarelli M, Radziejewski C, Fujimori T, Correia I (2012) Increased serum clearance of oligomannose species present on a human IgG1 molecule. MAbs 4:509–520
Morris MJ, Divgi CR, Pandit-Taskar N, Batraki M, Warren N, Nacca A, Smith-Jones P, Schwartz L, Kelly WK, Slovin S, Solit D, Halpern J, Delacruz A, Curley T, Finn R, O’Donoghue JA, Livingston P, Larson S, Scher HI (2005) Pilot trial of unlabeled and indium-111-labeled anti-prostate-specific membrane antigen antibody J591 for castrate metastatic prostate cancer. Clin Cancer Res 11:7454–7461
Battelli MG, Buonamici L, Bolognesi A, Stirpe F (1994) In vivo and in vitro uptake of an anti-CD30/saporin immunotoxin by rat liver parenchymal and nonparenchymal cells. Hepatology 20:940–947
Herbertson RA, Tebbutt NC, Lee F-T, MacFarlane DJ, Chappell B, Micallef N, Lee S-T, Saunder T, Hopkins W, Smyth FE, Wyld DK, Bellen J, Sonnichsen DS, Brechbiel MW, Murone C, Scott AM (2009) Phase I biodistribution and pharmacokinetic study of Lewis Y-targeting immunoconjugate CMD-193 in patients with advanced epithelial cancers. Clin Cancer Res 15:6709–6715
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gorovits, B., Krinos-Fiorotti, C. Proposed mechanism of off-target toxicity for antibody–drug conjugates driven by mannose receptor uptake. Cancer Immunol Immunother 62, 217–223 (2013). https://doi.org/10.1007/s00262-012-1369-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-012-1369-3