Abstract
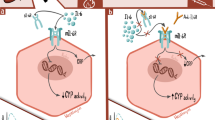
Interleukin 6 (IL-6) plays a central role in the immunopathogenesis of rheumatoid arthritis (RA) and tocilizumab [TCZ] (an anti-IL-6 receptor antibody) has been shown to be effective in the treatment of the condition. As up-regulation of IL-6 reduces the activity of cytochrome P450 (CYP) enzymes, blockade of this cytokine may enhance CYP function. This may lead to reduced bioavailability of CYP-metabolized drugs. Due to the increasing use of TCZ, we undertook a systematic literature review to explore such interactions. Our search was conducted in MEDLINE, EMBASE, Web of Science, FDA and EMEA websites for in vitro and in vivo studies, clinical trials and reviews mentioning TCZ and CYP on the basis of the title and abstract. Appropriate articles were further screened based on full-text review to select only those reporting IL-6, TCZ and their potential interaction with CYP-metabolized drugs. Two in vitro studies showed that TCZ-reversed IL-6 induced reduction of CYP isozymes. CYP3A4 mRNA expression was most reduced by IL-6 followed by CYP2C9 and CYP2C19. This change was prevented with TCZ. Three clinical studies investigated the interaction showing simvastatin (CYP3A4 substrate) bioavailability reduced by TCZ and omeprazole bioavailability was decreased by TCZ-induced CYP2C19 activity. The bioavailability of dextromethorphan (CYP2D6 and CYP3A4 substrates) was shown to be unaffected by TCZ treatment. The observed increase in CYP isozyme activity by TCZ is of clinical relevance as the bioavailability of the CYP isozyme substrates were decreased in vivo. As CYP3A4 is the isozyme responsible for the largest proportion of drug metabolism, it is probable that the bioavailability of other drugs may be reduced by TCZ. Thus, clinicians should exercise caution when co-prescribing TCZ and CYP-metabolized drugs. More studies are required to investigate this interaction further.
Similar content being viewed by others
References
Wolfe F, Rasker JJ, Boers M, Wells GA, Michaud K (2007) Minimal disease activity, remission, and the long-term outcomes of rheumatoid arthritis. Arthritis Rheum 57:935–942
Emery P, Keystone E, Tony HP et al (2008) IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 67:1516–1523
Patel A, Moreland L (2010) Interleukin-6 inhibition for treatment of rheumatoid arthritis: a review of tocilizumab therapy. Drug Des Devel Ther 4:4263–4278
Kishimoto T (2006) Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther 8:S2
Abdel-Razzak Z, Loyer P, Fautrel A, Gautier JC, Corcos L, Turlin B, Beaune P, Guillouzo A (1993) Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol 44:707–715
Rivory LP, Slaviero KA, Clarke SJ (2002) Hepatic cytochrome P450 3A drug metabolism is reduced in cancer patients who have an acute-phase response. Br J Cancer 87:277–280
Frye R, Virginia M, Schneider RN, Carole S (2002) Plasma levels of TNF-α and IL-6 are inversely related to cytochrome P450-dependent drug metabolism in patients with congestive heart failure. J Card Fail 8:315–319
Zhang X, Schmitt C, Grange S, Terao K, Miya K, Kivitz A, Marino M (2009) Disease-drug interaction studies of tocilizumab with cytochrome P450 substrates in vitro and in vivo. Clin Pharmacol Ther 85:0009–9236
Aitken A, Morgan E (2007) Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos 35:1687–1693
Schmitt C, Kuhn B, Zhang X, Kivitz AJ, Grange S (2011) Disease-drug-drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther 89:735–740
Terao K, Tsuru T, Suzaki M, Ishida Y, Amamoto T, Amamoto H, Higuchi S, Nishimoto N (2010) Drug-disease interaction study of tocilizumab in patients with rheumatoid arthritis—IL-6 signal inhibition normalised cytochrome P-450 enzymes expression which was reduced by inflammation. Int J Rheum Dis 13:1756–1841
European Medicines Agency. RoActemra (tocilizumab). Summary of product characteristics. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000955/WC500054890.pdf
Aithal G, Day C, Kesteven P, Daly A (1999) Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. The Lancet 353:717–719
Skolnick JL, Stoler BS, Katz DB, Anderson WH (1976) Rifampin, oral contraceptives and pregnancy. JAMA 236:1382
Ling S, Jamali F (2009) The effect of infliximab on hepatic cytochrome P450 and pharmacokinetics of verapamil in rats with pre-adjuvant arthritis: a drug-disease and drug–drug interaction. Basic Clin Pharmcol Toxicol 105:1742–7835
Conflict of interest
Andrew Östör has received support from (including attendance at conferences), undertakes clinical trials and acts as a consultant to Roche, Chugai, Schering-Plough/MSD, Abbott, Wyeth, BMS, GSK, MerckSorono and UCB. Soohan Kim and Muhammad K. Nisar have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S., Östör, A.J.K. & Nisar, M.K. Interleukin-6 and cytochrome-P450, reason for concern?. Rheumatol Int 32, 2601–2604 (2012). https://doi.org/10.1007/s00296-012-2423-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-012-2423-3