Abstract
Background
Amikacin is an aminoglycoside commonly used in intensive care units for the treatment of patients with life-threatening Gram-negative infections. Although aminoglycosides are extensively used, the accurate determination of their optimal dosage is complicated by marked intra- and interindividual variability in intensive care unit patients. Amikacin pharmacokinetics have been described in numerous studies over the past 25 years.
Objective
This review presents a synthesis of the population pharmacokinetic models for amikacin described in critically ill patients. The objective was to determine whether there was a consensus on a structural model and which covariates had been identified.
Methods
A literature search was conducted from the PubMed database, from its inception up until December 2015, using the following terms: ‘amikacin’, ‘pharmacokinetic(s)’, ‘population’, ‘model(ling)’ and ‘nonlinear mixed effect’. Articles were excluded if they were not pertinent. The reference lists of all selected articles were also evaluated.
Results
Ten articles were included in this review: pharmacokinetics of amikacin were described by a one-compartment or a two-compartment model. Various covariates were tested, but only two (creatinine clearance and total body weight) were included in almost all of the described models. After inclusion of these covariates, the interindividual variability (range) in clearance and the volume of distribution were 44.4 % (28.2–69.4 %) and 31.3 % (8.1–44.7 %), respectively. The residual variability (range) was around 21.0 % (9.0–31.0 %), using a proportional model, and for a combined model (proportional/additive), the median (range) values were 0.615 mg/L (0.2–1.03 mg/L) and 29.2 % (26.8–31.6 %).
Conclusion
This review highlights the different population pharmacokinetic models for amikacin developed in critically ill patients over the past decades and proposes relevant information for clinicians and researchers. To optimize amikacin dosage, this review points out the relevant covariates according to the target population. In a population of critically ill patients, dose optimization mainly depends on creatinine clearance and total body weight. New pharmacokinetic population studies could be considered, with new covariates of interest to be tested in model building and to further explain variability. Another future perspective could be external evaluation of previously published models.
Similar content being viewed by others
Amikacin has recently regained importance in the care of critically ill patients. |
There is wide intra- and interindividual variability influenced by pathophysiological conditions in critically ill patients. |
What are the factors that influence the variability of amikacin pharmacokinetic parameters in critically ill patients? |
1 Introduction
Aminoglycosides are commonly used in intensive care units (ICUs) for the treatment of patients with life-threatening Gram-negative infections. Despite widespread bacterial resistance to several groups of antimicrobial agents, amikacin remains one of the more frequently prescribed aminoglycosides in this population. Combination therapy with beta-lactam/amikacin has been recommended following recent guidelines and expert opinion on the treatment of sepsis, especially in patients with septic shock or suspected Pseudomonas aeruginosa infection [1, 2]. Amikacin optimal dosing is highly variable and depends on the site and severity of the infection, as well as on the susceptibility of the organism [3]. This drug exhibits a bactericidal effect related to its concentration (concentration-dependent killing). Once-daily administration to maximize its concentration-dependent effect and post-antibiotic effect is widely accepted as the standard regimen in general ward patients, as well as in ICU patients. Previous clinical studies have shown that a ratio of 10 or more between the concentration achieved 1 h (C 1h) after the beginning of a 30-min infusion and the minimal inhibitory concentration (MIC) for the bacteria responsible for the infection is predictive of therapeutic success [4].
Although aminoglycosides are extensively used, accurate determination of their optimal dosage is complicated by marked intra- and interindividual pharmacokinetic variability. In addition, the pharmacokinetic behaviour of a drug is known to be influenced by pathophysiological conditions [5]. In critically ill patients (with sepsis, trauma, etc.), amikacin disposition is altered by decreased protein binding, administration of multiple drugs and organ failure [6–11]. Unfortunately, antibiotic dosing regimens used in ICU rarely take into account pharmacokinetic modifications induced by pathophysiological changes, and an empirical dosing strategy of amikacin is inadequate in this population. It is therefore of interest to explore all of the approaches used to predict and control the pharmacokinetic variability of this drug in this patient population and propose individualized dosing regimens.
A narrow therapeutic index, potential adverse events and important variability have reinforced the role of therapeutic drug monitoring (TDM) of this drug. A definite benefit of TDM has been demonstrated for aminoglycosides, and therapy should be initiated as soon as possible [12]. Indeed, early achievement of an optimal C 1h/MIC ratio may have an impact on clinical and microbiological responses. TDM in critically ill patients allows optimized dosing in the presence of severely deranged pharmacokinetics [13]. Indeed, doses administered to critically ill patients are associated with decreased rates of achievement of peak concentration and area under the concentration–time curve targets. Although minimizing the likelihood of toxicity using TDM is important in critically ill patients, dose adaptation to avoid under-dosing and to maximize efficacy is also valuable [14].
This has made it necessary to more carefully examine the factors that influence the variability in amikacin pharmacokinetics. There have been many pharmacokinetic studies in critically ill patient populations to address this issue. In particular, nonlinear mixed-effects modelling, a commonly used population-based modelling approach, has been used to identify covariates that could influence the dose–concentration relationship. A population pharmacokinetic approach allows Bayesian dose estimation and adaptation according to population pharmacokinetic parameters and the estimated variability in a specific population. Population pharmacokinetic modelling was first introduced in 1972 by Sheiner et al. Thirty years after its introduction, population pharmacokinetic approaches have become a reference method for drug evaluation and dose adaptation [15].
This review presents a synthesis of the population pharmacokinetic analyses carried out for amikacin in critically ill patients. The objective was to determine whether there was a consensus on a structural model, which covariates have been identified and which covariates remain to be investigated.
2 Methods
2.1 Adherence to PRISMA Principles
We followed the principles of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement to guide the quality assessment of this review. The PRISMA statement helps to improve the reporting of systematic reviews with a checklist [16].
2.2 Inclusion Criteria
We included all described pharmacokinetic population models for amikacin. The articles were accepted if they met the following inclusion criteria:
-
Studied populations: critically ill adult patients.
-
Treatment: amikacin (intravenous).
-
Pharmacokinetic analysis: modelling by a population approach.
2.3 Exclusion Criteria
Articles were excluded if they were reviews or methodology articles, if the analysis did not use population pharmacokinetic modelling or if they were population studies without mixed-effects model analysis.
2.4 Search Strategy
A literature search was conducted in the PubMed database, from its inception up until December 2015, using the following terms: (‘amikacin’ AND ‘pharmacokinetics’ AND ‘critically ill patients’) OR (‘amikacin’ AND ‘pharmacokinetics’ AND ‘population’ AND ‘critically ill patients’) OR (‘amikacin’ AND ‘pharmacokinetics’ AND ‘model(ling)’ AND ‘critically ill patients’) OR (‘amikacin’ AND ‘pharmacokinetics’ AND ‘nonlinear mixed effect model’ AND ‘critically ill patients’). Moreover, additional studies were identified from the reference lists of the selected papers. The search was additionally limited to ‘English language’ and ‘clinical data’.
2.5 Data Extraction
The results of these investigations were closely evaluated, and articles were retained if they met the inclusion criteria. Pertinent articles were assessed, and the following data were extracted: year of publication, pathology, number of patients, number of samples, structural model, value and expression of pharmacokinetic parameters, included covariates, intra- and interindividual variability, and validation method.
Following Brendel et al. [17] and Tod et al. [18], the evaluation methods were divided into three categories according to increasing order of quality: basic internal methods (goodness-of-fit plots), advanced internal methods (bootstrap, cross-validation, Monte Carlo simulations, etc.) and external model evaluation.
3 Results
3.1 Trial Flow
A total of 36 studies were identified through PubMed database searching, with three additional articles identified via the reference lists of the selected articles. Thirty-nine articles were screened, and a total of 33 articles were first selected to have their full-text versions assessed for eligibility. Among these, 23 were excluded with reference to the inclusion and exclusion criteria. A total of ten articles were finally retained after three additional trials were added from reference scanning [19–28] (Fig. 1).
3.2 Study Characteristics
The ten studies described pharmacokinetic population models of amikacin and were published between 1995 and 2015. The studied populations consisted of critically ill patients with suspected Gram-negative infection, mainly septicaemia, pneumonia or severe trauma. Amikacin was administered as intermittent infusions according to different dosing regimens: once-daily dosing, twice-daily dosing or a dose interval adjusted according to a pharmacokinetic dosing method [19–28]. The doses administered ranged between 7.5 and 30 mg/kg/day (Table 1). The collected data resulted in therapeutic drug monitoring. Blood samples were collected after completion of the drug infusion (peak amikacin concentrations) and immediately before the start of amikacin administration (trough amikacin concentrations) [n = 5], or pharmacokinetic studies were done with full profiles of blood samples [n = 5] (Table 2).
3.3 Data Synthesis
Among the ten published models, amikacin population pharmacokinetics were described with either a one-compartment model [n = 5] or a two-compartment model [n = 5] (Table 2).
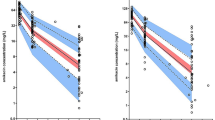
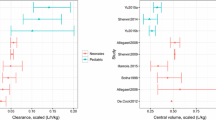
In all of the publications, several covariates were tested, and these are summarized in Table 3. The first criterion followed to retain a covariate in a model was a decrease in the objective function value (OFV) greater than 3.84 or 6.64 (p < 0.05 and p < 0.01, respectively) during the incorporation of covariates into the model (one by one). A second criterion was that the 95 % confidence interval of the estimated parameters did not include the parameter’s zero value. Differences in OFV between the basic model and the final model were specified in four studies (Table 3). The following covariates were selected as interindividual variability factors for clearance (CL) and volume of distribution (V): creatinine clearance (CLCR) [n = 9], total body weight [n = 5], positive end-expiratory pressure (PEEP), the ratio between the partial pressure of arterial oxygen and the fraction of inspired oxygen (PaO2/FiO2), oxygen extraction ratio, albumin concentration, use of catecholamines, ward, cirrhosis, cholecystitis category, trauma and sepsis. Table 4 summarizes the mean values of the pharmacokinetic parameters for the one- and two-compartment models described in critically ill patients. The median (range) estimate of total clearance was 4.0 L/h (0.77–5.5 L/h) [n = 6]. The median (range) estimate of the central volume of distribution was 18.6 L (10.7–41.5 L) [n = 10]. The median (range) estimate of intercompartmental clearance was 5.2 L/h (4.4–12.1 L/h) [n = 3]. The median (range) estimate of the peripheral volume of distribution was 21.4 L (9.4–55.2 L) [n = 3]. Interindividual variability was modelled using a proportional model. The median (range) values for interindividual variability in total and intercompartmental clearance were 33 % (28–69 %) [n = 6] and 27 % (17–104 %) [n = 3], respectively. The median (range) values for interindividual variability in the central and peripheral volumes of distribution were 26 % (8–39 %) [n = 8] and 47 % (44–64 %) [n = 3], respectively. Interoccasion variability was not included or estimated in these selected studies. The median (range) of residual variability using a proportional model was 21 % (9–31 %) [n = 4], and for a combined model, the median (range) values were 0.6 mg/L (0.2–1.0 mg/L) and 19 % (10–27 %) [n = 2].
All models were evaluated with internal or external methods (Table 2). Two authors used a basic internal evaluation. This evaluation most frequently used a calculation of indicators of the performance of prediction (bias and precision). Two authors used advanced internal evaluation, with a visual predictive check (500 simulations) and bootstrap (1000 simulations). In cases of external evaluation, six authors chose to test their model on a prospective group (between 5 and 108 patients). In numerous studies, bias and precision were calculated between measured and simulated concentrations, with simulations to evaluate model predictive performance. Simulations were also carried out in three studies in order to propose dosing regimen adaptation (Table 2). The probability of the concentration lying within the therapeutic range (maximum and minimum concentrations) was used as a target for the optimal dosage regimen design in two studies, while one author chose C 1h/MIC and AUC/MIC ratios as targets with a wide range of MICs.
4 Discussion
Amikacin is one of the most widely used antibiotics for the treatment of severe infections. There has been continued interest in describing amikacin pharmacokinetics for nearly 20 years, and several population pharmacokinetic models have been developed for patients with altered pharmacokinetic behaviour, including ICU patients.
However, only ten models have been described for amikacin in critically ill patients, using a nonlinear mixed-effects model [19–28]. Amikacin pharmacokinetics have been described by mono-exponential models as well as by bi-exponential models. Studies based on routine therapeutic drug monitoring samples, a practical solution with a noninvasive study, have mainly allowed the design of one-compartment models with an imprecise estimation of the volume of distribution, whereas full profile sampling has mostly allowed the design of two-compartment models. Moreover, the results expressed by these different models have led to similar estimations of pharmacokinetic parameters. The median values (range) of the clearance and volume of distribution of amikacin were 4.0 L/h (0.77–5.5 L/h) and 18.6 L (10.7–41.5 L), respectively. In this specific patient population, the disposition of amikacin is altered by an increased volume of distribution and reduced clearance as a result of leaky capillaries and organ failure [6–11]. Indeed, Garraffo et al. showed that clearance was approximately 7.0 L/h and the volume of distribution was approximately 11.0 L in healthy volunteers receiving amikacin doses between 7.5 and 15 mg/kg [29]. Three out of ten models described in this population contained fewer than 35 patients, which could limit the identification of a significant covariate [23, 24, 26]. Nevertheless, the models described in this population were able to identify some covariates with an effect on interindividual variability. Indeed, pharmacokinetic modifications and wide variability were described in this population.
Several pathophysiological changes occurring in ICU patients with sepsis can affect drug pharmacokinetic behaviour [30]. The cardiovascular, renal, pulmonary and hepatic systems can be affected by critical illness. Many dysfunctions of the cardiovascular system could be the origin of pharmacokinetic modifications, especially ‘third spacing’ (i.e. a significantly increased interstitial volume due to the administration of large volumes of resuscitation fluids in response to the resulting hypotension) [31]. An increased volume of distribution may reduce the peak concentrations of drugs, which may be problematic for antibiotics such as amikacin, which exhibit concentration-dependent effects. Concerning the renal system, many of the commonly used antibiotics in critically ill patients are subject to renal clearance; therefore, alterations in renal function affect concentrations of those antibiotics. With regard to the pulmonary system, pneumonia is the most common infection in critically ill patients and is an important cause of morbidity and mortality in ICU patients [32]. The antibiotic concentration in epithelial lining fluid may determine therapeutic success [33]. For hydrophilic antibiotics, such as amikacin, some authors suggest that higher doses should be used in patients with severe nosocomial pneumonia to optimize epithelial lining fluid concentrations [34, 35]. Finally, hepatic dysfunction can also affect critically ill patients; this dysfunction may cause a decrease in drug metabolism and clearance, but few data are available for this population. These pathophysiological changes leading to pharmacokinetic modifications could be included in the final model as covariates. A significant decrease in OFV and/or a significant decrease in the interindividual variability in clearance were obtained with creatinine clearance [19–27]. The effect of creatinine clearance on amikacin clearance was present in nine models. Indeed, amikacin, which has low level of protein binding (<20 %) and a molecular weight of 582.6, is mainly eliminated via the renal route [36]. Highly water-soluble antibiotics, such as amikacin, distribute primarily into the extracellular fluid compartment and are eliminated almost entirely by the kidneys via glomerular filtration; thus, modifications in renal function should directly affect the drug’s clearance [37, 38]. The relationship between total body weight and the volume of distribution was described in five studies, and administered amikacin doses are commonly adjusted to total body weight [19, 22, 24, 26, 27]. Increased cardiac and interstitial fluid shifts in sepsis result in a larger volume of distribution, which may reduce plasma antibiotic levels [39]. Specifically, the increase in the volume of distribution in septic patients has been attributed to hypoalbuminaemia and the resultant decrease in oncotic pressure, which may progress with a shift in body water from the intervascular to the extravascular space [40]. One model included serum albumin concentrations as an expression of the volume of distribution [26]. As stated previously, the cardiovascular system can be affected in critically ill patients. Some authors included other covariates that concerned cardiac outcomes. Indeed, PEEP, PaO2/FiO2 and the oxygen extraction ratio were included in different models and significantly reduced variability [19, 26]. Indeed, mechanical ventilation with PEEP is a well-documented cause of reductions in the cardiac index, hepatic and renal flow, the glomerular filtration rate and urine flow [41, 42]. In another model, disease severity was also included and use of catecholamines was added to the amikacin clearance estimation [26]. Finally, different comorbidities were also added in a few models (trauma, sepsis, cirrhosis, cholecystitis) [22, 27, 28]. Thus, trauma increased amikacin clearance, whereas sepsis increased the volume of distribution. Both observations had been found previously in studies on another aminoglycoside in specific ICU populations [43–45].
Regarding concomitant therapy, only three studies specified co-treatments and only two studies mentioned the names of other administered drugs [19, 21, 27]. In these two studies, amikacin was combined with a broad-spectrum beta-lactam (imipenem, meropenem, piperacillin, ceftazidime or cefepime) [19, 21]. Only one study tested concomitant therapy as a covariate, but it was not retained in the final model [19]. Critically ill patients receive multiple medications, and it is difficult to consider them as covariates. Nevertheless, analysis of frequent treatments (as part of clinical protocols) could be considered, especially when concomitant therapy could affect the pharmacokinetics of amikacin. Despite the number of models presented, some unexplained interindividual and residual variabilities remain. According to the different presented studies, the main covariates were tested, although a more accurate assessment of concomitant treatment would be useful. Furthermore, the day of drug administration and patient evolution are important data in this population, and they could help decrease variability. Indeed, significant intrasubject fluctuations in the volume of distribution of amikacin throughout treatment were observed in these different studies, and various physiological changes in critically ill patients were widely described [37]. Interoccasion variability was not included in the presented models. This variability could be included in new models to decrease the estimated interindividual pharmacokinetic and/or pharmacodynamic variances. However, pharmacokinetic/pharmacodynamic parameters in critically ill patients can be modified for each administration, so interoccasion variability is often difficult to estimate in this specific population. In spite of important unexplained variability in the different presented models, their results improve the knowledge of amikacin pharmacokinetics in critically ill patients and are helpful for designing simulations in order to optimize dosing. In many studies, simulations were carried out only in order to evaluate their model (bias and precision). Dosing adaptation was proposed in only three studies [19, 22, 27]. The proposed dosing regimens showed wide heterogeneity and could not be compared, because of different concentration targets [19, 22, 27].
Given the relatively small number of studies on population pharmacokinetic modelling of amikacin in critically ill patients, together with the small number of dose recommendations, new pharmacokinetic population studies could be considered. New covariates of interest could be tested in model building and may allow more of the variability to be explained. Antibiotic coadministration and systematic co-treatments in critically ill patients (sedation, gastric protection, etc.) could be tested. Furthermore, the pharmacogenetics of drug transporters could be an approach for future studies. Another perspective could be external evaluation of previously published models. It would also be interesting to continue research on the pharmacokinetics of amikacin to improve future models and further reduce variability, as well as to upgrade the care of critically ill patients treated with amikacin.
5 Conclusion
After many years of dosing antibiotics in critically ill patients, using a ‘one dose fits all’ strategy, there is a strong rationale to move to an individualized dosing approach. The population approach allows pharmacokinetic characterization of drugs in a target population, evaluation of the associated interpatient and residual variabilities, and identification of covariates affecting such variability.
Understanding the variability associated with the pharmacokinetics and identifying subpopulations with special features can provide clinicians with relevant information regarding dose individualization.
From a clinical perspective, this review advances relevant information for clinicians and researchers concerning the pharmacokinetics of amikacin. To optimize amikacin dosage, this review points out the relevant covariates according to the target population.
References
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi:10.1007/s00134-012-2769-8 [Epub 2013 Jan 30].
Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519–27.
Begg EJ, Barclay ML. Aminoglycosides—50 years on. Br J Clin Pharmacol. 1995;39:597–603.
Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155(1):93–9.
Zaske DE. Aminoglycosides. In: Evans WE, Schentag JJ, Jusko WJ, eds. Applied pharmacokinetics. Principles of therapeutic drug monitoring, 3rd ed. Vancouver: Applied Therapeutics; 1992;14:1–47.
Roberts JA, Lipman J. Antibacterial dosing in intensive care: pharmacokinetics, degree of disease and pharmacodynamics of sepsis. Clin Pharmacokinet. 2006;45:755–73.
Pea F, Viale P, Furlanut M. Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin Pharmacokinet. 2005;44:1009–34.
Paepe De, Belpaire FM, Buylaert WA. Pharmacokinetic and pharmacodynamic considerations when treating patients with sepsis and septic shock. Clin Pharmacokinet. 2002;41:1135–51.
Power BM, Forbes AM, van Heerden PV, Ilet KF. Pharmacokinetics of drugs used in critically ill adults. Clin Pharmacokinet. 1998;34:25–56.
Mehrotra R, De Gaudio R, Palazzo M. Antibiotic pharmacokinetic and pharmacodynamic considerations in critical illness. Intensive Care Med. 2004;30:2145–56.
Bodenham A, Shelly MP, Park GR. The altered pharmacokinetics and pharmacodynamics of drugs commonly used in critically ill patients. Clin Pharmacokinet. 1988;14:347–73.
Duszynska W, Taccone FS, Hurkacz M, Kowalska-Krochmal B, Wiela-Hojenska A, Kubler A. Therapeutic drug monitoring of amikacin in septic patients. Crit Care. 2013;17:R165.
Roberts JA, Norris R, Paterson DL, Martin JH. Therapeutic drug monitoring of antimicrobials. Br J Clin Pharmacol. 2012;73(1):27–36. doi:10.1111/j.1365-2125.2011.04080.x.
Wong G, Sime FB, Lipman J, Roberts JA. How do we use therapeutic drug monitoring to improve outcomes from severe infections in critically ill patients? BMC Infect Dis. 2014;28(14):288. doi:10.1186/1471-2334-14-288 (Review).
Sheiner LB, Rosenberg B, Melmon KL. Modelling of individual pharmacokinetics for computer-aided drug dosage. Comput Biomed Res. 1972;5(5):411–59.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):1–6.
Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B, Girard P, Laffont CM, Mentre F. Are population pharmacokinetics and/or pharmacodynamic model adequately evaluated? A survey of the littérature from 2002 to 2004. Clin Pharmacokinet. 2007;46(3):221–34.
Tod M, Jullien V, Pons G. facilitation of drug evaluation in children by population methods and modeling. Clin Pharmacokinet. 2008;47(4):231–43.
Burdet C, Pajot O, Couffignal C, Armand-Lefevre L, Foucrier A, Laouenan C, Wolff M, Massias L, Mentre F. Population pharmacokinetics of single-dose amikacin in critically ill patients with suspected ventilator-associated pneumonia. Eur J Clin Pharmacol. 2015;71:75–83.
Matar MK, Al-lanqawi Y, Abdul-Malek K, Jelliffe R. Amikacin population pharmacokinetics in critically ill Kuwaiti patients. Biomed Res Int. 2013;2013:202818. doi:10.1155/2013/202818 Epub 2013 Jan 30.
Delattre IK, Musuamba FT, Nyberg J, Taccone FS, Laterre PF, Verbeeck RK, Jacobs F, Wallemacq PE. Population pharmacokinetic modeling and optimal sampling strategy for Bayesian estimation of amikacin exposure in critically ill septic patients. Ther Drug Monit. 2010;32:749–56.
Romano S, De Catta M, Calvo V, Mendez E, Dominguez-Gil A, Lanao JM. Influence of clinical diagnosis in the population pharmacokinetics of amikacin in intensive care unit patients. Clin Drug Invest. 1998;15(5):435–44.
Joubert P, Bressolle F, Gouby A, Doucot PY, Saissi G, Gomeni R. A population approach to the forecasting of amikacin plasma and urinary levels using a prescribed dosage regimen. Eur J Drug Metab Pharmacokinet. 1999;24(1):39–46.
Bressolle F, Gouby A, Martinez JM, Joubert P, Saissi G, Guillaud R, Gomeni R. Population pharmacokinetics of amikacin in critically ill patients. AAC. 1996;40(7):1682–9.
Debord J, Pessis C, Voultoury JC, Marquet P, Lotfi H, Merle L, Lachatre G. Population pharmacokinetics of amikacin in intensive care unit patients studied by NPEM algorithm. Fundam Clin Pharmacol. 1995;9:57–61.
Lugo G, Castaneda-Hernandez G. Relationship between hemodynamic and vital support measures and pharmacokinetic variability of amikacin in critically ill patients with sepsis. Crit Care Med. 1997;25(5):806–11.
Jang SB, Lee YJ, Park MS, Song YG, Kim JH, Kim HK, Ahn BS, Park K. Population pharmacokinetics of amikacin in a Korean clinical population. Int J Clin Pharmacol Ther. 2011;49(6):371–81.
Lugo G, Castaneda-Hernandez G. Amikacin Bayesian forecasting in critically ill patients with sepsis and cirrhosis. Ther Drug Monit. 1997;19:271–6.
Garraffo R, Drugeon HB, Dellamonica P, Bernard E, Lapalus P. Determination of optimal dosage regimen for amikacin in healthy volunteers by study of pharmacokinetics and bactericidal activity. Antimicrob Agents Chemother. 1990;34(4):614–21.
Taccone FS, Laterre PF, Spapen H, Dugernier T, Delattre I, Layeux B, De Backer D, Wittebole X, Wallemacq P, Vincent JL, Jacobs F. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care. 2010;14(2):R53.
van der Poll T. Immunotherapy of sepsis. Lancet Infect Dis. 2001;1(3):165–74 (Review).
Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K, EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–9. doi:10.1001/jama.2009.1754.
Rodvold KA, Yoo L, George JM. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antifungal, antitubercular and miscellaneous anti-infective agents. Clin Pharmacokinet. 2011;50(11):689–704. doi:10.2165/11592900-000000000-00000 (Review).
Boselli E, Breilh D, Rimmelé T, Poupelin JC, Saux MC, Chassard D, Allaouchiche B. Plasma and lung concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia. Intensive Care Med. 2004;30(5):989–91 Epub 2004 Feb 24.
Lodise TP, Sorgel F, Melnick D, Mason B, Kinzig M, Drusano GL. Penetration of meropenem into epithelial lining fluid of patients with ventilator-associated pneumonia. Antimicrob Agents Chemother. 2011;55(4):1606–10. doi:10.1128/AAC.01330-10 Epub 2011 Feb 7.
Regeur L, Colding H, Jensen H, Kampmann JP. Pharmacokinetics of amikacin during hemodialysis and peritoneal dialysis. AAC. 1997;11(2):214–8.
Zaske DE, Strate RG, Kohls PR. Amikacin pharmacokinetics: wide interpatient variation in 98 patients. J Clin Pharmacol. 1991;31(2):158–63.
Mathews A, Bailie GR. Clinical pharmacokinetics, toxicity and cost effectiveness analysis of aminoglycosides and aminoglycoside dosing services. J Clin Pharm Ther. 1987;12(5):273–91.
Van Dalen R, Vree TB. Pharmacokinetics of antibiotics in critically ill patients. Intensive Care Med. 1990;16(3):S235–8.
Oparaoji EC, Cornwell EE 3rd, Hekmat E, Lum Cheong R, Adir JS, Siram S. Aminoglycoside volume of distribution in postoperative patients with septic shock. Clin Pharm. 1993;12(2):131–4.
Berry AJ. Respiratory support and renal function. Anesthesiology. 1981;55:655–67.
Richard C, Berdeaux A, Delion F, Riou B, Rimailho A, Giudicelli JF, Auzépy P. Effect of mechanical ventilation on hepatic drugs pharmacokinetics. Chest. 1986;90:837–41.
Triginer C, Izquierdo I, Fernández R, Rello J, Torrent J, Benito S, Net A. Gentamicin volume of distribution in critically ill septic patients. Intensive Care Med. 1990;16:303–6.
Dasta JF, Amstrong DK. Variability in aminoglucoside pharmacokinetics in critically ill surgical patients. Crit Care Med. 1988;16:327–30.
Watling SM, Dasta JF. Aminoglycoside dosing considerations in intensive care unit patients. Ann Pharmacother. 1993;27:351–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used in the preparation of this review.
Conflict of interest
Amélie Marsot, Romain Guilhaumou, Camille Riff and Olivier Blin have no conflicts of interest that are relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Marsot, A., Guilhaumou, R., Riff, C. et al. Amikacin in Critically Ill Patients: A Review of Population Pharmacokinetic Studies. Clin Pharmacokinet 56, 127–138 (2017). https://doi.org/10.1007/s40262-016-0428-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0428-x