-
PDF
- Split View
-
Views
-
Cite
Cite
R. Wenzel, A. Del Favero, C. Kibbler, T. Rogers, C. Rotstein, J. Mauskopf, S. Morris, H. Schlamm, P. Troke, A. Marciniak, Economic evaluation of voriconazole compared with conventional amphotericin B for the primary treatment of aspergillosis in immunocompromised patients, Journal of Antimicrobial Chemotherapy, Volume 55, Issue 3, March 2005, Pages 352–361, https://doi.org/10.1093/jac/dkh535
Close - Share Icon Share
Abstract
Objective: The objective of this study was to conduct an economic evaluation of voriconazole compared with conventional amphotericin B deoxycholate (CAB) using data from a recently reported randomized comparative trial in patients with various underlying immunosuppressive conditions. This trial demonstrated the superiority of voriconazole in terms of clinical response, survival and safety when used as primary therapy for invasive aspergillosis.
Methods: A decision analytic model was designed using an expert panel and populated primarily with efficacy and resource utilization data collected prospectively during the clinical trial. The analysis was carried out from the perspective of the health care system and all costs are reported in 2002 US dollars.
Results: Average total treatment costs per patient were 10% lower in the voriconazole arm ($30 664) than in the CAB arm ($34 144), resulting from reduced consumption of hospital resources and fewer changes in antifungal therapy. In the base case analysis, voriconazole provided an average saving of $3481 per treated patient, resulted in a lower cost per survivor ($43 310 versus $58 971) and a lower cost per successfully treated patient ($58 100 versus $108 124) compared with CAB. Sensitivity analyses demonstrated that the cost savings observed were maintained over a wide range of alternative values for both unit costs and resource utilization, including length of hospital stay, time spent in intensive care units, bed day costs and the cost of lipid formulations of amphotericin B.
Conclusion: Incremental cost-effectiveness analysis indicated the dominance of voriconazole because of both lower costs and greater efficacy.
Introduction
Invasive aspergillosis (IA) is a complex and frequently multifocal infection that is emerging as a major clinical problem.1,2 During the last 20 years in the USA, the frequency of invasive mycoses including aspergillosis has risen significantly to become the seventh leading cause of death in the infectious disease category.3,4 This rise corresponds to a substantial increase in the number of immunocompromised patients.5
The most recent US National Centre for Health Statistics (NCHS) multiple-cause-of-death records indicate that mortality associated specifically with IA had risen 357% between 1980 and 1997.4 The case fatality for patients with IA is approximately 58% and is even greater in patients with central nervous system infection or disseminated aspergillosis (88%) or with diffuse invasive pulmonary disease (60%).6 High mortality as a result of IA is also seen in patients who have undergone bone marrow transplantation (87%), liver transplantation (92–100%)7,8 or those with underlying HIV infection (86%).6 An earlier study of bone marrow transplant patients also estimated the mortality directly attributable to IA to be high at 62% (CI95 44–80%).9
Few studies have specifically estimated the economic consequences of IA, but the associated financial burden is consistently high. In 1998, the total first year medical costs in the USA were estimated to be $674 million,10 with an average annual cost per patient of $72 792. Of this, $36 867 was the extra hospitalization cost attributable to IA. The additional expenditure of treating aspergillosis is eight times higher than the per person average annual expenditure of $4094 on health care in the USA.11
Conventional amphotericin B deoxycholate (CAB) and voriconazole are the only two antifungal medications currently approved as primary therapy for invasive aspergillosis infection in the USA. For decades, CAB has been the standard first line treatment for life-threatening invasive fungal infections, including IA.12 However, response is suboptimal in severely immunocompromised patients13,14 with an overall success rate of 34%.15 Its effective use is also limited because of a variety of toxic effects, the most significant being nephrotoxicity and acute infusion-related adverse events such as fever, chills and rigors.16,17
Second line treatments for IA in patients who are refractory or intolerant to CAB include lipid-based formulations of amphotericin, itraconazole and caspofungin. Evidence suggests that lipid formulations may be as efficacious as CAB but with fewer and less severe adverse effects18–20 and current practice is to initiate treatment with lipid formulations when clinically necessary.13 However, the use of these products places a greater economic burden on institutional drug budgets due to their high acquisition costs. Both caspofungin and itraconazole have proven efficacy against aspergillosis infection but their comparative efficacy versus CAB has not been prospectively demonstrated.
The dose-limiting toxicity associated with CAB and the limited clinical proof of success with other agents indicate a substantial unmet medical need in this area.
Voriconazole is a new second-generation antifungal triazole with a broad spectrum of activity against fungal pathogens. In the Global Comparative Aspergillosis (GCA) study, the largest comparative trial conducted in IA,21 the efficacy and safety of voriconazole (n=144) for the primary treatment of acute IA were compared with CAB (n=133). This study demonstrated that initial therapy with voriconazole resulted in a better clinical response (P < 0.0001) (complete or partial resolution of all attributable symptoms, signs, radiographic/bronchoscopic abnormalities present at baseline) and improved survival (P < 0.02). Discontinuations as a result of adverse events were also less frequently seen in the voriconazole group (P=0.02). The acquisition cost of voriconazole however was higher than CAB, so the relative cost-effectiveness of this new treatment option needed to be demonstrated.
The objective of this study was to conduct an economic evaluation of voriconazole compared with CAB as primary therapy for invasive aspergillosis. The analysis was carried out using a decision analytical model. The model examined the economic consequences of initiating therapy with either voriconazole or CAB to treat one episode of invasive aspergillosis over 12 weeks. The perspective taken was that of the health care system. As the main burden of care for IA has been shown to be in the hospital sector,10 the analysis focused on the direct costs of inpatient and outpatient hospital care.
Materials and methods
Development of the model
The clinical management of IA is complex and individuals treated with commonly used antifungal agents may require several changes in therapy either because of a lack of efficacy or the occurrence of treatment-related toxic effects. In order to undertake an economic evaluation, a simplified decision tree based on the clinical management of immunocompromised patients, with definite or probable IA, was developed.
An independent international panel comprising five clinicians from the USA, Canada, UK and Italy, with specialist knowledge and clinical experience with systemic antifungal therapy, was convened. Individuals completed a detailed questionnaire on treatment practices to help ensure that the economic model developed reflected clinical practice. A modified Delphi technique was used to obtain consensus.22 The panel also advised on additional analyses of GCA data where required. In a subsequent face-to-face meeting they validated the structure, time horizon and assumptions used. Other specific inputs are detailed below.
Model structure
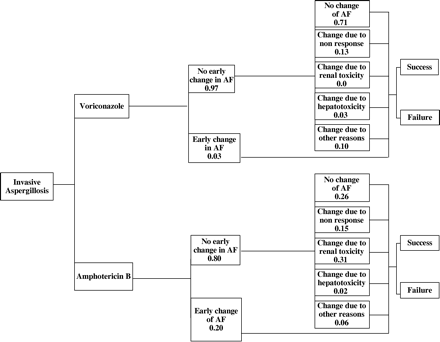
The decision tree designed to estimate the costs and consequences of a typical length of a treated IA episode (12 weeks) is shown in Figure 1. Following initial treatment with either voriconazole or CAB, patients who had an inadequate response to therapy or who experienced severe toxicity could be changed to any other licensed antifungal therapy (OLAT). The model provided six possible treatment pathways depending on whether or not a patient was changed from initial antifungal (AF) treatment and, if so, for what reason. The pathway options were as follows: no change from initial AF therapy, early change in AF therapy due to severe toxicity (within the first 4 days), change due to non-response, change due to major renal toxicity, change due to major hepatotoxicity and change for any other reason.
For all treatment pathways, the end points of treatment success (cured or improved) or treatment failure (deterioration or no improvement) at 12 weeks were as defined in the GCA21 study. Survival at 84 days was also assessed.
Direct hospital medical costs associated with the treatment of IA were included in the model. These represented the costs of diagnosis and monitoring of the infection, acquisition of antifungal agents, prophylaxis, treatment and monitoring of AF-related adverse events (drugs and tests). Also included were hospital resources, comprising the length of stay (LOS) in an intensive care unit (ICU) and other non-ICU settings, and outpatient care.
Model inputs
The model developed by the panel was populated with data derived primarily from the GCA study.21 This included clinical success rates, morbidity and mortality, the probability of changing from initial AF therapy, the duration of initial therapy, the reasons for changing AF therapy, details of other OLATs used in each pathway as well as the health care resources utilized. Data sources are detailed in the relevant tables (Tables 1–3).
Table 1 summarizes the probabilities of successful treatment, all causes of mortality and change from initial AF therapy to OLAT.
For patients who were not changed from initial antifungal therapy, all the data for the model was directly taken from the GCA study.21 This included the duration of treatment with initial AF therapy and OLATs, hospital stay (ICU and total) and the number of day hospital and outpatient visits. The duration of antifungal therapy and hospitalization for both voriconazole and CAB arms was censored at 84 days to ensure the same duration of observation.
The average duration of intravenous (iv) treatment with CAB in patients who were not changed from initial therapy was 21 days. In this group, 20% stepped down to oral itraconazole and on average received an additional 7 days of oral therapy. Those who remained on voriconazole received a mean of 14 days of iv treatment followed by 50 days of oral therapy.
Patients who remained on CAB had a mean hospital stay of 18 days compared with 23 days for those who continued on voriconazole, reflecting the increased survival observed in the latter group. The average length of stay in intensive care was 4 days for both groups. Patients who stayed on initial therapy with CAB made three hospital day visits and those continuing on voriconazole made four. Both groups made two outpatients visits.
For patients who were changed to an OLAT, data on resource use and duration of antifungal therapy before the switch were also taken from the GCA, as well as the proportion of each OLAT used in the different pathways. For those who were changed to an OLAT as a result of early severe toxicity, the mean duration of initial AF treatment in both arms was 3 days. For those who changed to an OLAT as a result of non-response, renal toxicity or hepatotoxicity, the average duration of treatment on initial therapy was 16 days for CAB and 26 days for voriconazole.
The proportion of days spent on different OLATs used in the base case for each of the five pathways where patients were changed from initial AF therapy is shown in Table 2.
Due to the very low number of patients in each OLAT pathway in the GCA study, expert opinion was necessary to ensure consistency of some resource utilization data with clinical practice and with the aggregated results of the GCA trial. This opinion was informed by the clinical trial data and based on the assumptions outlined below.
A full course of OLAT treatment was assumed for all changes in therapy. Patients changed to CAB or to liposomal amphotericin B were assumed to have a duration of treatment with these drugs equal to the length of therapy with CAB for non-switch patients in the GCA study. Similarly, patients changed to itraconazole were assumed to have a duration of treatment with itraconazole equal to the length of non-switch voriconazole therapy in the GCA study.
Patients changed to CAB were assumed to have spent an additional 18 days in hospital including four in ICU and to have made three hospital day visits and two outpatient consultations. Those who changed to any other OLAT were assumed to have spent an additional 23 days in hospital including six in ICU and to have made four hospital day visits and two outpatient consultations.
Other resource use data not available from the GCA study21 were provided by the panel. These data included tests for the diagnosis and monitoring of IA as well as daily doses for drugs used to moderate adverse events resulting from antifungal therapy. The latter included acetaminophen, chlorpheniramine, and meperidine/pethidine administered with all iv antifungals; hydrocortisone administered with iv CAB; granulocyte–macrophage colony stimulating factor and piperacillin/tazobactam for patients with neutropenia. For both CAB and voriconazole, the frequency of complete blood counts, renal and liver function tests and chest X-rays were estimated at two per week, CT scans at one per week and bronchoalveolar lavage and fungal culture at once during the 12 week time period.
All of the unit costs used in the model are shown in Table 3. Medication costs for antifungal treatments and drugs prescribed for the prophylaxis and treatment of antifungal adverse events were obtained from the American Hospital Formulary Service Red Book.23 Medicare fees for service unit costs, including monitoring for side effects, screening for fungal infection, and hospital outpatient care were obtained from the complete St Anthony's Resource-Based Relative-Value Scale (RBRVS).24
Model calculations
The model was used to generate a ‘weighted’ average cost for all patients initially treated with voriconazole or CAB. This was done by calculating average costs for each of the six treatment pathways for both arms in the decision tree and then weighting the costs according to the proportional distribution of patients in each treatment pathway (Table 1).
The cost of antifungal therapy for each pathway was determined by adding the cost of initial AF therapy to the cost of subsequent therapy with OLAT. The cost of initial AF therapy was calculated as a proportion of the cost of a full course of voriconazole or CAB, according to the number of days of therapy, before changing to OLAT. The entire period before the switch was costed as hospital days.
The cost of OLAT therapy in each pathway was obtained by calculating the total cost for individual OLATs and weighting these according to the proportion of days spent on different OLATs (Table 2).
The cost per successfully treated patient for voriconazole or CAB was calculated using the weighted average cost in each arm divided by the probability of treatment success in each arm. The cost per survivor for voriconazole or CAB was calculated using the weighted average cost in each arm divided by the probability of survival in each arm. The calculation of the probability of survival used data from all patients who were alive at the end of 12 weeks irrespective of their outcome (treatment success, worsening IA, stable disease or an indeterminate response).
All costs were reported in 2002 US dollars. As costs and benefits of treating IA were examined over a 12 week period, no discounting of future costs or benefits was necessary.
Model assumptions
As observed in clinical practice and in the GCA study, patients who were switched from initial AF therapy may have had several consecutive changes in OLAT over 12 weeks (e.g. CAB to lipid amphotericin B to itraconazole). This is depicted as one change in the decision tree. The proportion of the different OLATs in each treatment pathway however has been defined as a proportion of total days spent on all OLATs in the GCA study, thereby taking all switches in consideration when calculating the cost of treatment (Table 2).
Suspending treatment with CAB for more than 1 day was classified as a change in AF therapy. Patients who switched from CAB to itraconazole because the investigator wanted to step-down from iv infusion to oral treatment remained in the ‘no change from initial therapy’ treatment pathway as did those who were switched from iv to oral voriconazole. The costs associated with these switches were included in the model.
An average value for resource use was calculated for each treatment pathway irrespective of whether the outcome was success or failure.
Sensitivity analyses
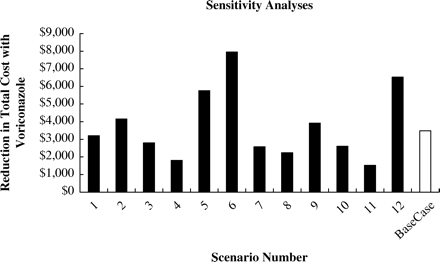
To evaluate the robustness of findings from the model, univariate sensitivity analyses were carried out to examine the impact of sequentially modifying the value of several key variables, including time to change from initial AF therapy, AF costs, and hospital length of stay (LOS) (Figure 2). The analyses also evaluated the impact of excluding the resource use where estimates were provided by the expert panel.
To reflect the usage of different formulations of liposomal amphotericin B (LAB) in clinical practice, the impact of using either 100% liposomal amphotericin B (AmBisome) or 100% amphotericin B complex (Abelcet) was examined. As hospitals may purchase antifungals at discounted prices, the effect of decreasing the list price of LABs by 50% was also evaluated.
The average LOS used in the model for patients who do not change from initial AF therapy was higher for voriconazole than for CAB due to significantly better survival, so the impact on the average cost of using the same LOS (total and ICU) for both treatments was examined.
The impact of using OLAT data from the 150-602 protocol of the GCA study,21 which included mainly North American patients, instead of pooled data from all patients, was studied.
In addition to the above, two scenarios relating to the assumptions for duration of additional treatment and hospital stay in patients switched to different OLAT regimes were tested. For duration of hospital stay, these assumed either a decrease from the base case to 4 ICU days and 10 other hospital days (low case) or an increase to 8 ICU days and 25 other hospital days (high case). For duration of additional treatment with OLATs, these assumed either a decrease from the base case to 14 days of iv treatment with liposomal amphotericin and 37 days of itraconazole treatment (7 days of iv followed by 30 days of oral therapy) (low case) or an increase to 42 days of treatment with liposomal amphotericin B (28 days of iv therapy followed by 14 days of oral itraconazole) and 91 days of treatment with itraconazole (21 days of iv followed by 70 days of oral therapy) (high case).
Results
Average treatment costs
The average weighted cost for the voriconazole arm was lower ($30 664) than for the CAB arm ($34 144), representing an average cost saving of $3481 (10%) per treated patient (Table 4). Early severe toxicity and major renal toxicity were the main cost drivers in the CAB group.
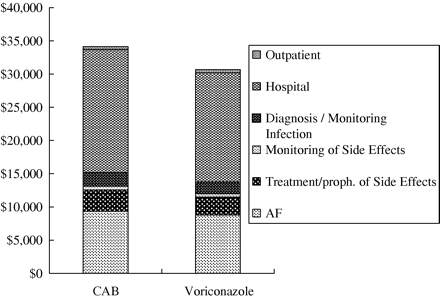
The relative contribution of different cost components for both arms is shown in Figure 3.
Cost per survivor and per success
The probability of survival at 12 weeks was higher in the voriconazole arm than in the CAB arm (0.71 versus 0.58) and the average cost per survivor was lower in the voriconazole arm ($43 310 versus $58 971). The average cost per successfully treated patient was also lower with voriconazole than with CAB ($58 100 versus $108 124).
Incremental cost effectiveness
Using base case assumptions, voriconazole was dominant to CAB as first line therapy. It had both a lower total treatment cost and was more efficacious in terms of the number of patients successfully treated and the number surviving at 12 weeks (Table 5). The difference in average treatment costs therefore provided a better indicator of product value than an incremental cost effectiveness ratio.
Sensitivity analyses
All 12 scenarios examined showed cost savings for the voriconazole arm confirming that the dominance of voriconazole is robust even when changing key variables (Figure 2).
The model was sensitive to changes in ICU bed day costs (scenario 4) and the time to switch to OLATs (scenarios 5 and 6). Reducing the cost of LAB (scenarios 8, 10, 11) also had a large impact on cost differentials. However, even when the lowest priced LAB (Abelcet) was assumed as the only LAB (scenario 10), the cost-savings associated with using voriconazole still remained substantial at $2236 (6.5%).
When using OLAT switch data from the 150-602 protocol of the GCA study,21 including mainly North-American patients (scenario 12) and where a much higher use of LAB than in the pooled GCA study (65% versus 28% of OLAT days) was observed, the cost-saving of using voriconazole increased to $6532.
The impact of changing ICU and hospital days following a change to OLAT resulted in cost savings in favour of voriconazole of $1239 (low case) and $6008 (high case), respectively. The impact of changing length of therapy with OLATs produced cost savings also in favour of voriconazole of $1426 (low case) and $5540 (high case), respectively.
Discussion
This is the first economic study that compares voriconazole with CAB as primary therapy for definite or probable IA in immunocompromised patients. The base case analysis demonstrated that using voriconazole provided an average cost saving of $3481 per treated patient. In addition, voriconazole resulted in a lower cost per survivor (difference of $15 661) and a lower cost per successfully treated patient (difference of $50 024). The findings from this study together with the previously reported results of the GCA study21 demonstrated that voriconazole provided a saving of direct inpatient and outpatient hospital treatment costs and was a better treatment option in terms of treatment success, survival and safety.
The direct attributable cost of treating IA is high. In this study, the average weighted cost, irrespective of the outcome, was estimated to be $30 664 for voriconazole and $34 144 for CAB. Because of differences in methodology and time periods covered, these results are not directly comparable with findings for IA from other recent studies.10,11 However, they are in line with previous estimates of attributable costs. In 1996, Dasbach et al. approximated the cost per patient for hospitalization to be US $51 779.11 In 1998, hospitalization costs related to the treatment of aspergillosis were estimated at US $36 867, the highest attributable per patient hospitalization cost of all fungal infections.10
The model in this study was populated primarily with resource use data from the GCA study,21 a prospective randomized controlled trial. The findings are therefore considerably more reliable than those from previous studies evaluating the economics of presumed25–27 or confirmed fungal infection.28,29 The GCA study had a substantial number of patients in each group, and the criteria used for diagnosis were contemporaneous, practical, validated, standardized, reproducible and similar to those agreed by the EORTC Invasive Fungal Infections Co-operative Group/Mycoses Study Group Consortium.30 The care received by patients during the study reflected current clinical practice and all patients initially randomized to therapy were followed up for the entire 12 week study period. Although some of the resource utilization data were estimated by the panel, these resources accounted for only 17% of total costs and excluding them in the sensitivity analysis did not alter the cost savings seen when initiating treatment with voriconazole. While some values for duration of length of stay for patients who switched had to be estimated by expert opinion, the total weighted LOS in each arm as calculated by the model was compared with the LOS in the GCA study. The difference was less than 0.2 days for total and less than 0.1 for ICU hospital stay.
For consistency between clinical and resource utilization data, the usage of OLAT was taken from the pooled data of the GCA study.21 However, when the analysis included a usage of OLAT more closely reflecting US practices, the cost savings with voriconazole nearly doubled.
Economic evaluations often use life years gained as the main measure of outcome. However, without long-term survival data for patients with the different underlying conditions represented in this model, it was not possible to provide reliable estimates of life years gained from this study.
Sensitivity analyses showed that the cost savings demonstrated by the model were most sensitive to ICU and general ward bed day costs, the acquisition cost of LAB and the time interval between commencing initial AF treatment and changing to an OLAT. When the cost of general ward days was increased more than three-fold to ICU bed day costs, voriconazole remained cost saving. If Abelcet was considered as the only LAB used, or the daily cost of LAB decreased by 50%, or the daily dose of AmBisome reduced to 3 mg/kg, voriconazole still remained cost saving.
The cost savings achieved with voriconazole compared with CAB increased significantly if the time on initial therapy was increased for voriconazole or decreased for CAB. In the GCA study,21 investigators were encouraged to maintain patients on CAB for at least 14 days, and the median usage was 10 days (range 1–84).21
This study has demonstrated that current drug switching practices can increase the average cost of treatment with CAB by more than 100% and that early switching due to severe toxicity or major renal toxicity were the main cost drivers in the CAB group. Part of this increased cost resulted from the usage of antifungals such as LAB, which have much higher acquisition costs, and treating AF-related toxicity. These findings are in line with a previous study showing that the occurrence of renal toxicity associated with the use of CAB increased the cost of treatment by $29 823.25
Despite the less than optimal safety profile of CAB,31 it often remains the preferred first line option for the treatment of fungal infections because of its broad spectrum of activity and low acquisition cost. Lipid formulations of amphotericin B, developed to improve the risk–benefit ratio,32 are being increasingly used33 both as first line and second line treatment, however the daily acquisition costs for the lipid complex (Abelcet) and liposomal amphotericin B (AmBisome) are all much higher than those for CAB or voriconazole.32 For example, at 2002 prices, the daily cost for a 65 kg patient receiving AmBisome is $1318.80. This is 66 times higher than for CAB and at least five times more costly than voriconazole iv whereas Abelcet at a daily cost of $920 is 46 times higher than CAB and at least three times more costly than voriconazole iv.23
There are no cost effectiveness studies that have directly compared CAB or voriconazole with one of the lipid formulations in IA. A cost effectiveness study28 of AmBisome versus CAB, based on retrospective data from 58 transplant recipients with systemic mycoses, indicated that healthcare costs were saved by the reduced usage of other drugs, fewer re-operations and a reduced need for treating adverse events. However, these savings offset only 36% of the acquisition costs of AmBisome. A cost-effectiveness study comparing liposomal amphotericin B to CAB for empirical therapy in febrile neutropenic patients demonstrated superior efficacy for AmBisome but found that total treatment costs for AmBisome were higher than for CAB ($48 962 versus $43 183, P=0.022). This was despite savings in hospital costs related to lower renal toxicity (18.7% versus 33.7%).25 The only comparative study published to date of CAB versus LAB in invasive aspergillosis demonstrated lower renal toxicity (25% versus 49%, P=0.002) but there were no differences in efficacy or survival between the two study arms.19 The above findings indicate that the economic advantage of using voriconazole would be maintained or even increased when compared to different lipid formulations. However, a review of the literature did not provide sufficient details on resource use with these products to allow a meaningful comparison using this model.
The model used a number of conservative assumptions in favour of CAB treatment. Only one switch in antifungal therapy was assumed for both CAB and voriconazole arms, however, the trend was for a higher number of switches in the CAB arm (1.8 versus 1.6) in the GCA study.21 While this was taken into account for the drug costs calculations, the other resource use implications of multiple switches were not considered in the model. The resources used in screening and monitoring and the drugs used to minimize adverse events during treatment were assumed to be the same for both treatment arms although expert opinion indicated that there is less use of these resources in patients treated with voriconazole. These factors may therefore overestimate the costs for voriconazole treatment and underestimate those associated with CAB treatment.
Censoring of data at 84 days in both arms was conducted to ensure the same duration of observation in both arms and therefore comparability over the 12 week period. While this period was considered long enough to cover the majority of aspergillosis-related costs, it could bias against CAB treatment because voriconazole resulted in longer survival and likely increased costs associated with the treatment of underlying conditions.
In the GCA study,21 there was a significantly higher proportion of patients with proven IA in the voriconazole arm compared with the CAB arm. As clinical success rates were lower in patients with proven disease (44.8% versus 59.7%), the relative efficacy of voriconazole to CAB is likely to be underestimated. Therefore, the differences in cost per successfully treated patient and cost per survivor may also be underestimated by this model in favour of CAB.
Substantial variations in cost have been demonstrated for different sub-groups.10,11 In the GCA study,21 the number of patients with a specific underlying condition in the various treatment pathways was too small to provide reliable estimates of the impact of a patient's underlying condition on the average weighted cost of treatment. However, the voriconazole and CAB groups were well matched and the clinical outcomes for voriconazole were also superior to those observed with CAB in all of the sub-groups of patients.21 The differences in resource utilization between voriconazole and CAB arms in the various sub-groups were also consistent with, and comparable to the differences in the total population [Pfizer data on file, 2003]. It is therefore likely that using voriconazole in any population of patients with a diagnosis of IA would result in cost savings when compared with using CAB.
The burden of treating IA is high, accounting for 26% ($674 million) of total annual expenditure on fungal infections in 1998.10 Over half of this burden is the cost of hospitalization. In a US setting, this study demonstrated the opportunity for savings in direct costs driven by a reduction in hospital costs and additional antifungal medication usage. Therefore, in addition to saving lives, using voriconazole as primary therapy for IA could potentially reduce overall healthcare expenditure.
Decision tree for the treatment of definite/probable invasive aspergillosis. The probabilities were obtained from the GCA study.21
Sensitivity analyses. Scenario 1, use voriconazole total hospital length of stay of 23 days for CAB; 2, increase CAB ICU bed days from 4 to 5 days; 3, use ICU daily bed cost for general ward bed day costs; 4, use general ward daily bed cost for ICU bed day costs; 5, use CAB time of 16 days to change from initial AF therapy to OLAT for voriconazole time to change from initial AF therapy; 6, use voriconazole time of 26 days to change from initial AF therapy to OLAT for CAB time to change from initial AF therapy; 7, exclude costs estimated by the expert panel; 8, use dose of 3 mg/kg for AmBisome instead of 5 mg/kg; 9, use AmBisome as only lipid formulation of amphotericin B ($1318.8 per day); 10, use Abelcet as only lipid formulation of amphotericin B ($920 per day); 11, decrease the cost of lipid formulations of amphotericin B by 50% ($592.94 per day); 12, Use OLAT switch data from 150-602 protocol including mainly North American patients.
Efficacy and rates of change from initial randomized antifungal therapy – base case
| . | Probabilities . | . | ||
|---|---|---|---|---|
| . | CAB arm . | voriconazole arm . | ||
| No change from initial randomized therapy (IRT)a | 0.263 | 0.715 | ||
| Changes in AF therapy due to: | ||||
| early severe toxicity | 0.195 | 0.028 | ||
| non-response | 0.150 | 0.132 | ||
| major renal toxicity | 0.308 | 0.000 | ||
| major hepatotoxicity | 0.023 | 0.028 | ||
| other reason | 0.060 | 0.097 | ||
| All changes to OLAT | 0.736 | 0.285 | ||
| DRC global success at week 12 (including OLAT) | 0.316 | 0.528b | ||
| Failure (including deaths) | 0.684 | 0.472 | ||
| All-cause mortality at week 12 | 0.421 | 0.292c | ||
| . | Probabilities . | . | ||
|---|---|---|---|---|
| . | CAB arm . | voriconazole arm . | ||
| No change from initial randomized therapy (IRT)a | 0.263 | 0.715 | ||
| Changes in AF therapy due to: | ||||
| early severe toxicity | 0.195 | 0.028 | ||
| non-response | 0.150 | 0.132 | ||
| major renal toxicity | 0.308 | 0.000 | ||
| major hepatotoxicity | 0.023 | 0.028 | ||
| other reason | 0.060 | 0.097 | ||
| All changes to OLAT | 0.736 | 0.285 | ||
| DRC global success at week 12 (including OLAT) | 0.316 | 0.528b | ||
| Failure (including deaths) | 0.684 | 0.472 | ||
| All-cause mortality at week 12 | 0.421 | 0.292c | ||
Source: data analysis of GCA study (12 week MITT population).21 CAB, amphotericin B deoxycholate; DRC, Data Review Committee.
Includes iv CAB with step down to oral itraconazole.
P < 0.0001.
P < 0.02.
Efficacy and rates of change from initial randomized antifungal therapy – base case
| . | Probabilities . | . | ||
|---|---|---|---|---|
| . | CAB arm . | voriconazole arm . | ||
| No change from initial randomized therapy (IRT)a | 0.263 | 0.715 | ||
| Changes in AF therapy due to: | ||||
| early severe toxicity | 0.195 | 0.028 | ||
| non-response | 0.150 | 0.132 | ||
| major renal toxicity | 0.308 | 0.000 | ||
| major hepatotoxicity | 0.023 | 0.028 | ||
| other reason | 0.060 | 0.097 | ||
| All changes to OLAT | 0.736 | 0.285 | ||
| DRC global success at week 12 (including OLAT) | 0.316 | 0.528b | ||
| Failure (including deaths) | 0.684 | 0.472 | ||
| All-cause mortality at week 12 | 0.421 | 0.292c | ||
| . | Probabilities . | . | ||
|---|---|---|---|---|
| . | CAB arm . | voriconazole arm . | ||
| No change from initial randomized therapy (IRT)a | 0.263 | 0.715 | ||
| Changes in AF therapy due to: | ||||
| early severe toxicity | 0.195 | 0.028 | ||
| non-response | 0.150 | 0.132 | ||
| major renal toxicity | 0.308 | 0.000 | ||
| major hepatotoxicity | 0.023 | 0.028 | ||
| other reason | 0.060 | 0.097 | ||
| All changes to OLAT | 0.736 | 0.285 | ||
| DRC global success at week 12 (including OLAT) | 0.316 | 0.528b | ||
| Failure (including deaths) | 0.684 | 0.472 | ||
| All-cause mortality at week 12 | 0.421 | 0.292c | ||
Source: data analysis of GCA study (12 week MITT population).21 CAB, amphotericin B deoxycholate; DRC, Data Review Committee.
Includes iv CAB with step down to oral itraconazole.
P < 0.0001.
P < 0.02.
Proportion of days spent on other licensed antifungal therapies (OLAT) in each pathwaya – base case
| Early change from initial AF therapy due to severe toxicity | CAB arm (n=26) | Voriconazole arm (n=4) |
| CAB | 2.1%b | 26.2% |
| Lipid AB | 40.9% | 73.8% |
| Itraconazole | 48.8% | 0.0% |
| CAB + itraconazole | 0.1% | 0.0% |
| Lipid AB + itraconazole | 7.8% | 0.0% |
| Lipid AB + flucytosine | 0.3% | 0.0% |
| Change from initial AF therapy due to non-response | CAB arm (n=20) | Voriconazole arm (n=19) |
| CAB | 9.4%b | 30.6% |
| Lipid AB | 15.4% | 32.9% |
| Itraconazole | 51.3% | 27.7% |
| CAB + itraconazole | 11.0% | 5.2% |
| CAB + flucytosine | 0.0% | 2.4% |
| Lipid AB + itraconazole | 13.0% | 1.1% |
| Change from initial AF therapy due to severe renal toxicity | CAB arm (n=41) | Voriconazole arm (n=0) |
| CAB | 16.4%b | 0.0% |
| Lipid AB | 29.6% | 0.0% |
| Itraconazole | 50.4% | 0.0% |
| CAB + itraconazole | 0.4% | 0.0% |
| Lipid AB + itraconazole | 3.0% | 0.0% |
| Lipid AB + flucytosine | 0.1% | 0.0% |
| Change from initial AF therapy due to severe hepatotoxicity | CAB arm (n=3) | Voriconazole arm (n=4) |
| CAB | 62.9%b | 33.0% |
| Lipid AB | 24.3% | 2.4% |
| Itraconazole | 12.9% | 59.2% |
| Lipid AB + flucytosine | 0.0% | 5.3% |
| Change from initial AF therapy due to other reason | CAB arm (n=8) | Voriconazole arm (n=14) |
| CAB | 7.1%b | 8.4% |
| Lipid AB | 15.7% | 30.0% |
| Itraconazole | 76.9% | 61.3% |
| CAB + itraconazole | 0.3% | 0.0% |
| Early change from initial AF therapy due to severe toxicity | CAB arm (n=26) | Voriconazole arm (n=4) |
| CAB | 2.1%b | 26.2% |
| Lipid AB | 40.9% | 73.8% |
| Itraconazole | 48.8% | 0.0% |
| CAB + itraconazole | 0.1% | 0.0% |
| Lipid AB + itraconazole | 7.8% | 0.0% |
| Lipid AB + flucytosine | 0.3% | 0.0% |
| Change from initial AF therapy due to non-response | CAB arm (n=20) | Voriconazole arm (n=19) |
| CAB | 9.4%b | 30.6% |
| Lipid AB | 15.4% | 32.9% |
| Itraconazole | 51.3% | 27.7% |
| CAB + itraconazole | 11.0% | 5.2% |
| CAB + flucytosine | 0.0% | 2.4% |
| Lipid AB + itraconazole | 13.0% | 1.1% |
| Change from initial AF therapy due to severe renal toxicity | CAB arm (n=41) | Voriconazole arm (n=0) |
| CAB | 16.4%b | 0.0% |
| Lipid AB | 29.6% | 0.0% |
| Itraconazole | 50.4% | 0.0% |
| CAB + itraconazole | 0.4% | 0.0% |
| Lipid AB + itraconazole | 3.0% | 0.0% |
| Lipid AB + flucytosine | 0.1% | 0.0% |
| Change from initial AF therapy due to severe hepatotoxicity | CAB arm (n=3) | Voriconazole arm (n=4) |
| CAB | 62.9%b | 33.0% |
| Lipid AB | 24.3% | 2.4% |
| Itraconazole | 12.9% | 59.2% |
| Lipid AB + flucytosine | 0.0% | 5.3% |
| Change from initial AF therapy due to other reason | CAB arm (n=8) | Voriconazole arm (n=14) |
| CAB | 7.1%b | 8.4% |
| Lipid AB | 15.7% | 30.0% |
| Itraconazole | 76.9% | 61.3% |
| CAB + itraconazole | 0.3% | 0.0% |
Source: data analysis of the GCA study (12-week MITT population).21n represents the number of patients in the group from which the distribution of days on the listed OLATs was taken. Figures may not sum to 100% because of rounding.
Percentage of OLAT days from the GCA study (12-week MITT population), calculated as the total days on each OLAT from first change in AF therapy until end of trial divided by the total number of days on all OLATs from time of first change in AF therapy until end of trial.
Patients discontinued from CAB for more than 24 h or receiving OLAT, which was later discontinued and then changed back to CAB.
Proportion of days spent on other licensed antifungal therapies (OLAT) in each pathwaya – base case
| Early change from initial AF therapy due to severe toxicity | CAB arm (n=26) | Voriconazole arm (n=4) |
| CAB | 2.1%b | 26.2% |
| Lipid AB | 40.9% | 73.8% |
| Itraconazole | 48.8% | 0.0% |
| CAB + itraconazole | 0.1% | 0.0% |
| Lipid AB + itraconazole | 7.8% | 0.0% |
| Lipid AB + flucytosine | 0.3% | 0.0% |
| Change from initial AF therapy due to non-response | CAB arm (n=20) | Voriconazole arm (n=19) |
| CAB | 9.4%b | 30.6% |
| Lipid AB | 15.4% | 32.9% |
| Itraconazole | 51.3% | 27.7% |
| CAB + itraconazole | 11.0% | 5.2% |
| CAB + flucytosine | 0.0% | 2.4% |
| Lipid AB + itraconazole | 13.0% | 1.1% |
| Change from initial AF therapy due to severe renal toxicity | CAB arm (n=41) | Voriconazole arm (n=0) |
| CAB | 16.4%b | 0.0% |
| Lipid AB | 29.6% | 0.0% |
| Itraconazole | 50.4% | 0.0% |
| CAB + itraconazole | 0.4% | 0.0% |
| Lipid AB + itraconazole | 3.0% | 0.0% |
| Lipid AB + flucytosine | 0.1% | 0.0% |
| Change from initial AF therapy due to severe hepatotoxicity | CAB arm (n=3) | Voriconazole arm (n=4) |
| CAB | 62.9%b | 33.0% |
| Lipid AB | 24.3% | 2.4% |
| Itraconazole | 12.9% | 59.2% |
| Lipid AB + flucytosine | 0.0% | 5.3% |
| Change from initial AF therapy due to other reason | CAB arm (n=8) | Voriconazole arm (n=14) |
| CAB | 7.1%b | 8.4% |
| Lipid AB | 15.7% | 30.0% |
| Itraconazole | 76.9% | 61.3% |
| CAB + itraconazole | 0.3% | 0.0% |
| Early change from initial AF therapy due to severe toxicity | CAB arm (n=26) | Voriconazole arm (n=4) |
| CAB | 2.1%b | 26.2% |
| Lipid AB | 40.9% | 73.8% |
| Itraconazole | 48.8% | 0.0% |
| CAB + itraconazole | 0.1% | 0.0% |
| Lipid AB + itraconazole | 7.8% | 0.0% |
| Lipid AB + flucytosine | 0.3% | 0.0% |
| Change from initial AF therapy due to non-response | CAB arm (n=20) | Voriconazole arm (n=19) |
| CAB | 9.4%b | 30.6% |
| Lipid AB | 15.4% | 32.9% |
| Itraconazole | 51.3% | 27.7% |
| CAB + itraconazole | 11.0% | 5.2% |
| CAB + flucytosine | 0.0% | 2.4% |
| Lipid AB + itraconazole | 13.0% | 1.1% |
| Change from initial AF therapy due to severe renal toxicity | CAB arm (n=41) | Voriconazole arm (n=0) |
| CAB | 16.4%b | 0.0% |
| Lipid AB | 29.6% | 0.0% |
| Itraconazole | 50.4% | 0.0% |
| CAB + itraconazole | 0.4% | 0.0% |
| Lipid AB + itraconazole | 3.0% | 0.0% |
| Lipid AB + flucytosine | 0.1% | 0.0% |
| Change from initial AF therapy due to severe hepatotoxicity | CAB arm (n=3) | Voriconazole arm (n=4) |
| CAB | 62.9%b | 33.0% |
| Lipid AB | 24.3% | 2.4% |
| Itraconazole | 12.9% | 59.2% |
| Lipid AB + flucytosine | 0.0% | 5.3% |
| Change from initial AF therapy due to other reason | CAB arm (n=8) | Voriconazole arm (n=14) |
| CAB | 7.1%b | 8.4% |
| Lipid AB | 15.7% | 30.0% |
| Itraconazole | 76.9% | 61.3% |
| CAB + itraconazole | 0.3% | 0.0% |
Source: data analysis of the GCA study (12-week MITT population).21n represents the number of patients in the group from which the distribution of days on the listed OLATs was taken. Figures may not sum to 100% because of rounding.
Percentage of OLAT days from the GCA study (12-week MITT population), calculated as the total days on each OLAT from first change in AF therapy until end of trial divided by the total number of days on all OLATs from time of first change in AF therapy until end of trial.
Patients discontinued from CAB for more than 24 h or receiving OLAT, which was later discontinued and then changed back to CAB.
Unit costs – base case
| Item . | Unit . | Daily cost for 65 kg patient (US$) . | ||
|---|---|---|---|---|
| Drugs | ||||
| Amphotericin B deoxycholate | 1.0 mg/kg iva | 20.00 | ||
| AmBisome | 5 mg/kg iva | 1318.80 | ||
| Abelcet | 5 mg/kg iva | 920.00 | ||
| Lipid formulation of amphotericin B in GCA study (2/3 AmBisome, 1/3 Abelcet) | 1185.90 | |||
| Itraconazole | 400 mg injectiona | 295.15 | ||
| Itraconazole | 400 mg orala | 31.11 | ||
| Flucytosine | 100 mg/kga | 91.27 | ||
| Voriconazole | 12 mg/kgb | 340.00 | ||
| Voriconazole | 8 mg/kgb | 255.00 | ||
| Voriconazole | 200 mg oral bidb | 50.00 | ||
| Acetaminophen/paracetamol | 1 g orala | 0.05 | ||
| GM-CSF | 300 μg injectiona | 183.54 | ||
| Chlorpheniramine | 10 mg injectiona | 0.40 | ||
| Meperidine/pethidine injection | 50 mg/mL injectiona | 0.67 | ||
| Hydrocortisone 25 mg injection | 50 mg/mLa | 3.60 | ||
| Piperacillin/tazobactam | 4 g/0.5 g tdsa | 184.80 | ||
| Monitoring tests | Units | Unit cost (US$) | ||
| Complete blood count | 1 | 19.55 | ||
| Renal function | 1 | 6.15 | ||
| Liver function | 1 | 36.00 | ||
| Screening tests | ||||
| Chest X-ray | 1 | 35.88 | ||
| CT scan | 1 | 271.13 | ||
| Bronchoalveolar lavage | 1 | 151.31 | ||
| Fungal culture (non blood) | 1 | 19.55 | ||
| Hospitalization/outpatient care | ||||
| Inpatient (ICU) | 1 day | 1348.00c | ||
| Inpatient (infectious diseases) | 1 day | 375.00d | ||
| Outpatient (infectious diseases) | 1 day | 90.50 | ||
| Outpatient visit | 1 25-min visit | 57.77 | ||
| Item . | Unit . | Daily cost for 65 kg patient (US$) . | ||
|---|---|---|---|---|
| Drugs | ||||
| Amphotericin B deoxycholate | 1.0 mg/kg iva | 20.00 | ||
| AmBisome | 5 mg/kg iva | 1318.80 | ||
| Abelcet | 5 mg/kg iva | 920.00 | ||
| Lipid formulation of amphotericin B in GCA study (2/3 AmBisome, 1/3 Abelcet) | 1185.90 | |||
| Itraconazole | 400 mg injectiona | 295.15 | ||
| Itraconazole | 400 mg orala | 31.11 | ||
| Flucytosine | 100 mg/kga | 91.27 | ||
| Voriconazole | 12 mg/kgb | 340.00 | ||
| Voriconazole | 8 mg/kgb | 255.00 | ||
| Voriconazole | 200 mg oral bidb | 50.00 | ||
| Acetaminophen/paracetamol | 1 g orala | 0.05 | ||
| GM-CSF | 300 μg injectiona | 183.54 | ||
| Chlorpheniramine | 10 mg injectiona | 0.40 | ||
| Meperidine/pethidine injection | 50 mg/mL injectiona | 0.67 | ||
| Hydrocortisone 25 mg injection | 50 mg/mLa | 3.60 | ||
| Piperacillin/tazobactam | 4 g/0.5 g tdsa | 184.80 | ||
| Monitoring tests | Units | Unit cost (US$) | ||
| Complete blood count | 1 | 19.55 | ||
| Renal function | 1 | 6.15 | ||
| Liver function | 1 | 36.00 | ||
| Screening tests | ||||
| Chest X-ray | 1 | 35.88 | ||
| CT scan | 1 | 271.13 | ||
| Bronchoalveolar lavage | 1 | 151.31 | ||
| Fungal culture (non blood) | 1 | 19.55 | ||
| Hospitalization/outpatient care | ||||
| Inpatient (ICU) | 1 day | 1348.00c | ||
| Inpatient (infectious diseases) | 1 day | 375.00d | ||
| Outpatient (infectious diseases) | 1 day | 90.50 | ||
| Outpatient visit | 1 25-min visit | 57.77 | ||
Sources: Red Book,23 2002 Resource-Based Relative-Value Scale, Expert panel, MedTap, 2001 and Veenstra et al.,35 iv, intravenous; bid, twice a day; tds, three times a day.
Dose of drug from independent panel.
Product monograph.
MEDTAP, 2001.34
Veenstra et al.35
Unit costs – base case
| Item . | Unit . | Daily cost for 65 kg patient (US$) . | ||
|---|---|---|---|---|
| Drugs | ||||
| Amphotericin B deoxycholate | 1.0 mg/kg iva | 20.00 | ||
| AmBisome | 5 mg/kg iva | 1318.80 | ||
| Abelcet | 5 mg/kg iva | 920.00 | ||
| Lipid formulation of amphotericin B in GCA study (2/3 AmBisome, 1/3 Abelcet) | 1185.90 | |||
| Itraconazole | 400 mg injectiona | 295.15 | ||
| Itraconazole | 400 mg orala | 31.11 | ||
| Flucytosine | 100 mg/kga | 91.27 | ||
| Voriconazole | 12 mg/kgb | 340.00 | ||
| Voriconazole | 8 mg/kgb | 255.00 | ||
| Voriconazole | 200 mg oral bidb | 50.00 | ||
| Acetaminophen/paracetamol | 1 g orala | 0.05 | ||
| GM-CSF | 300 μg injectiona | 183.54 | ||
| Chlorpheniramine | 10 mg injectiona | 0.40 | ||
| Meperidine/pethidine injection | 50 mg/mL injectiona | 0.67 | ||
| Hydrocortisone 25 mg injection | 50 mg/mLa | 3.60 | ||
| Piperacillin/tazobactam | 4 g/0.5 g tdsa | 184.80 | ||
| Monitoring tests | Units | Unit cost (US$) | ||
| Complete blood count | 1 | 19.55 | ||
| Renal function | 1 | 6.15 | ||
| Liver function | 1 | 36.00 | ||
| Screening tests | ||||
| Chest X-ray | 1 | 35.88 | ||
| CT scan | 1 | 271.13 | ||
| Bronchoalveolar lavage | 1 | 151.31 | ||
| Fungal culture (non blood) | 1 | 19.55 | ||
| Hospitalization/outpatient care | ||||
| Inpatient (ICU) | 1 day | 1348.00c | ||
| Inpatient (infectious diseases) | 1 day | 375.00d | ||
| Outpatient (infectious diseases) | 1 day | 90.50 | ||
| Outpatient visit | 1 25-min visit | 57.77 | ||
| Item . | Unit . | Daily cost for 65 kg patient (US$) . | ||
|---|---|---|---|---|
| Drugs | ||||
| Amphotericin B deoxycholate | 1.0 mg/kg iva | 20.00 | ||
| AmBisome | 5 mg/kg iva | 1318.80 | ||
| Abelcet | 5 mg/kg iva | 920.00 | ||
| Lipid formulation of amphotericin B in GCA study (2/3 AmBisome, 1/3 Abelcet) | 1185.90 | |||
| Itraconazole | 400 mg injectiona | 295.15 | ||
| Itraconazole | 400 mg orala | 31.11 | ||
| Flucytosine | 100 mg/kga | 91.27 | ||
| Voriconazole | 12 mg/kgb | 340.00 | ||
| Voriconazole | 8 mg/kgb | 255.00 | ||
| Voriconazole | 200 mg oral bidb | 50.00 | ||
| Acetaminophen/paracetamol | 1 g orala | 0.05 | ||
| GM-CSF | 300 μg injectiona | 183.54 | ||
| Chlorpheniramine | 10 mg injectiona | 0.40 | ||
| Meperidine/pethidine injection | 50 mg/mL injectiona | 0.67 | ||
| Hydrocortisone 25 mg injection | 50 mg/mLa | 3.60 | ||
| Piperacillin/tazobactam | 4 g/0.5 g tdsa | 184.80 | ||
| Monitoring tests | Units | Unit cost (US$) | ||
| Complete blood count | 1 | 19.55 | ||
| Renal function | 1 | 6.15 | ||
| Liver function | 1 | 36.00 | ||
| Screening tests | ||||
| Chest X-ray | 1 | 35.88 | ||
| CT scan | 1 | 271.13 | ||
| Bronchoalveolar lavage | 1 | 151.31 | ||
| Fungal culture (non blood) | 1 | 19.55 | ||
| Hospitalization/outpatient care | ||||
| Inpatient (ICU) | 1 day | 1348.00c | ||
| Inpatient (infectious diseases) | 1 day | 375.00d | ||
| Outpatient (infectious diseases) | 1 day | 90.50 | ||
| Outpatient visit | 1 25-min visit | 57.77 | ||
Sources: Red Book,23 2002 Resource-Based Relative-Value Scale, Expert panel, MedTap, 2001 and Veenstra et al.,35 iv, intravenous; bid, twice a day; tds, three times a day.
Dose of drug from independent panel.
Product monograph.
MEDTAP, 2001.34
Veenstra et al.35
Weighted average cost of treatment
| . | CAB . | . | . | Voriconazole . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment arm . | weighta,ba,b . | cost (US$) . | weighted cost (US$)c . | weighta,ba,b . | cost (US$) . | weighted cost (US$)c . | ||||||
| No change from initial randomized therapy (IRT) | 0.263 | 15 561.85 | 4095.22 | 0.715 | 22 961.14 | 16 423.60 | ||||||
| Change in AF therapy | ||||||||||||
| early severe toxicity | 0.195 | 36 789.05 | 7191.84 | 0.028 | 39 126.65 | 1086.85 | ||||||
| non response | 0.150 | 42 829.06 | 6440.46 | 0.132 | 51 167.09 | 6751.21 | ||||||
| major renal toxicity | 0.308 | 42 744.75 | 13 176.95 | 0.000 | 0.00 | 0.00 | ||||||
| major hepatotoxicity | 0.023 | 36 800.85 | 830.09 | 0.028 | 46 217.13 | 1283.81 | ||||||
| other reason | 0.060 | 40 063.19 | 2409.82 | 0.097 | 52 644.68 | 5118.23 | ||||||
| Average patient total cost | 34 144.39 | 30 663.70 | ||||||||||
| . | CAB . | . | . | Voriconazole . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment arm . | weighta,ba,b . | cost (US$) . | weighted cost (US$)c . | weighta,ba,b . | cost (US$) . | weighted cost (US$)c . | ||||||
| No change from initial randomized therapy (IRT) | 0.263 | 15 561.85 | 4095.22 | 0.715 | 22 961.14 | 16 423.60 | ||||||
| Change in AF therapy | ||||||||||||
| early severe toxicity | 0.195 | 36 789.05 | 7191.84 | 0.028 | 39 126.65 | 1086.85 | ||||||
| non response | 0.150 | 42 829.06 | 6440.46 | 0.132 | 51 167.09 | 6751.21 | ||||||
| major renal toxicity | 0.308 | 42 744.75 | 13 176.95 | 0.000 | 0.00 | 0.00 | ||||||
| major hepatotoxicity | 0.023 | 36 800.85 | 830.09 | 0.028 | 46 217.13 | 1283.81 | ||||||
| other reason | 0.060 | 40 063.19 | 2409.82 | 0.097 | 52 644.68 | 5118.23 | ||||||
| Average patient total cost | 34 144.39 | 30 663.70 | ||||||||||
CAB, amphotericin B deoxycholate.
Weighting based on the proportional distribution of patients in each treatment arm.
For clarity the weights shown in the table have been rounded to the nearest decimal place.
Weighted costs have been calculated using actual weights.
Weighted average cost of treatment
| . | CAB . | . | . | Voriconazole . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment arm . | weighta,ba,b . | cost (US$) . | weighted cost (US$)c . | weighta,ba,b . | cost (US$) . | weighted cost (US$)c . | ||||||
| No change from initial randomized therapy (IRT) | 0.263 | 15 561.85 | 4095.22 | 0.715 | 22 961.14 | 16 423.60 | ||||||
| Change in AF therapy | ||||||||||||
| early severe toxicity | 0.195 | 36 789.05 | 7191.84 | 0.028 | 39 126.65 | 1086.85 | ||||||
| non response | 0.150 | 42 829.06 | 6440.46 | 0.132 | 51 167.09 | 6751.21 | ||||||
| major renal toxicity | 0.308 | 42 744.75 | 13 176.95 | 0.000 | 0.00 | 0.00 | ||||||
| major hepatotoxicity | 0.023 | 36 800.85 | 830.09 | 0.028 | 46 217.13 | 1283.81 | ||||||
| other reason | 0.060 | 40 063.19 | 2409.82 | 0.097 | 52 644.68 | 5118.23 | ||||||
| Average patient total cost | 34 144.39 | 30 663.70 | ||||||||||
| . | CAB . | . | . | Voriconazole . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment arm . | weighta,ba,b . | cost (US$) . | weighted cost (US$)c . | weighta,ba,b . | cost (US$) . | weighted cost (US$)c . | ||||||
| No change from initial randomized therapy (IRT) | 0.263 | 15 561.85 | 4095.22 | 0.715 | 22 961.14 | 16 423.60 | ||||||
| Change in AF therapy | ||||||||||||
| early severe toxicity | 0.195 | 36 789.05 | 7191.84 | 0.028 | 39 126.65 | 1086.85 | ||||||
| non response | 0.150 | 42 829.06 | 6440.46 | 0.132 | 51 167.09 | 6751.21 | ||||||
| major renal toxicity | 0.308 | 42 744.75 | 13 176.95 | 0.000 | 0.00 | 0.00 | ||||||
| major hepatotoxicity | 0.023 | 36 800.85 | 830.09 | 0.028 | 46 217.13 | 1283.81 | ||||||
| other reason | 0.060 | 40 063.19 | 2409.82 | 0.097 | 52 644.68 | 5118.23 | ||||||
| Average patient total cost | 34 144.39 | 30 663.70 | ||||||||||
CAB, amphotericin B deoxycholate.
Weighting based on the proportional distribution of patients in each treatment arm.
For clarity the weights shown in the table have been rounded to the nearest decimal place.
Weighted costs have been calculated using actual weights.
Results from the model of invasive aspergillosis
| . | CAB arm . | Voriconazole arm . | Difference . |
|---|---|---|---|
| Total average cost for all patients treated (successful and unsuccessful) | $34 144.39 | $30 633.70 | $3480.69 |
| Probability of successful treatment | 0.316 | 0.528 | –0.212a |
| Cost per successfully treated case | $108 123.91 | $58 099.65 | |
| Incremental cost per successful case | dominant | < $0 | |
| Probability of survival at week 12 | 0.579 | 0.708 | –0.129b |
| Cost per survivor | $58 971.32 | $43 310.31 | |
| Incremental cost per life saved | dominant | < $0 | |
| NNT to save one additional life | 7.75 | ||
| NNT to successfully treat one additional person | 4.80 |
| . | CAB arm . | Voriconazole arm . | Difference . |
|---|---|---|---|
| Total average cost for all patients treated (successful and unsuccessful) | $34 144.39 | $30 633.70 | $3480.69 |
| Probability of successful treatment | 0.316 | 0.528 | –0.212a |
| Cost per successfully treated case | $108 123.91 | $58 099.65 | |
| Incremental cost per successful case | dominant | < $0 | |
| Probability of survival at week 12 | 0.579 | 0.708 | –0.129b |
| Cost per survivor | $58 971.32 | $43 310.31 | |
| Incremental cost per life saved | dominant | < $0 | |
| NNT to save one additional life | 7.75 | ||
| NNT to successfully treat one additional person | 4.80 |
NNT, number needed to treat.
P < 0.001.
P=0.02.
Results from the model of invasive aspergillosis
| . | CAB arm . | Voriconazole arm . | Difference . |
|---|---|---|---|
| Total average cost for all patients treated (successful and unsuccessful) | $34 144.39 | $30 633.70 | $3480.69 |
| Probability of successful treatment | 0.316 | 0.528 | –0.212a |
| Cost per successfully treated case | $108 123.91 | $58 099.65 | |
| Incremental cost per successful case | dominant | < $0 | |
| Probability of survival at week 12 | 0.579 | 0.708 | –0.129b |
| Cost per survivor | $58 971.32 | $43 310.31 | |
| Incremental cost per life saved | dominant | < $0 | |
| NNT to save one additional life | 7.75 | ||
| NNT to successfully treat one additional person | 4.80 |
| . | CAB arm . | Voriconazole arm . | Difference . |
|---|---|---|---|
| Total average cost for all patients treated (successful and unsuccessful) | $34 144.39 | $30 633.70 | $3480.69 |
| Probability of successful treatment | 0.316 | 0.528 | –0.212a |
| Cost per successfully treated case | $108 123.91 | $58 099.65 | |
| Incremental cost per successful case | dominant | < $0 | |
| Probability of survival at week 12 | 0.579 | 0.708 | –0.129b |
| Cost per survivor | $58 971.32 | $43 310.31 | |
| Incremental cost per life saved | dominant | < $0 | |
| NNT to save one additional life | 7.75 | ||
| NNT to successfully treat one additional person | 4.80 |
NNT, number needed to treat.
P < 0.001.
P=0.02.
We acknowledge the assistance of Michael Ortiz, from RTI Health Solutions, Research Triangle Park, NC, USA in the data analysis. We also thank Yvonne Lis for assistance in preparing this manuscript. This study was funded by a grant from Pfizer Ltd., Worldwide Outcomes Research Group, UK.
References
Kontoyiannis, D. P & Bodey, G. P. (
Groll, A. H. & Walsh, T. J. (
Pinner, R. W., Teutsch, S. M., Simonsen, L. et al. (
McNeil, M. M., Nash, S. L., Hajjeh, R. A. et al. (
Lin, S.-J., Schranz, J. & Teutsch, S. M. (
Briegel, J., Forst, H., Spill, B. et al. (
Rabkin, J. M., Oroloff, S. L., Corless, C. L. et al. (
Pannuti, C., Gingrich, R., Pfaller, M. A. et al. (
Wilson, L. S., Reyes, C. M., Stolpman, M. et al. (
Dasbach, E. J., Davies, G. M. & Teutsch, S. M. (
Dismukes, W. E. (
Stevens, D. A., Kan, V. L., Judson, M. A. et al. (
Patterson, T. F., Kirkpatrick, W. R., White, M. et al. (
Al-Mohsen, I. & Hughes, W. T. (
Sheppard, D. & Lampiris, H. W (
Wingard, J. R., White, M. H., Anaissie, E. et al. (
Bowden, R., Chandrasekar, P., White, M. H. et al. (
Facts and Comparisons. (
Herbrecht, R., Denning, D. W., Patterson, T. F. et al. (
Bowling, A. Ed. (
Resource-Based Relative-Value Scale (RBRVS). (
Cagnoni, P., Walsh, T., Prendergast, T. et al. (
Annemans, L., Moeremans, K. & van Gool, R. (
Flynn, T. N., Kelsey, S. M., Hazel, D. L. et al. (
Persson, U., Tennvall, G. R., Andersson, S. et al. (
Dranitsaris, G., Phillips, P., Rotstein, C. et al. (
Ascioglu, S., Rex, J. H., de Pauw, B. et al. (
Gallis, H. A., Drew, R. H. & Pickard, W. W. (
Kaltenbach, B. L. & Ross, M. B. (
Walsh, T. J., Pappas, P., Winston, D. J. et al. (
Anon. (
Author notes
1Department of Internal Medicine, Medical College of Virginia, Virginia Commonwealth University, Old City Hall, 1001 East Broad Street, 4th Floor Suite 405, P.O. Box 980663, Richmond, VA 23298-0663; 6RTI Health Solutions, Research Triangle Park, NC; 8Pfizer Inc, New York, USA; 5Hamilton Health Sciences, Hamilton, Ontario, Canada; 2Institute of Internal Medicine and Oncology, University of Perugia, Perugia, Italy; 3Department of Medical Microbiology, Royal Free Hospital, London; 4Department of Infectious Diseases, Imperial College, Hammersmith Hospital, London; 7Department of Health Economics, Imperial College Management School, London; 9Pfizer Ltd, Sandwich, UK