Abstract
Background: Adverse drug events (ADEs) are a substantial cause of hospital admissions. However, little is known about the incidence, preventability and severity of ADEs resulting in emergency department visits. To address this issue, we conducted a prospective survey in emergency departments of French public hospitals.
Methods: This study was performed over two periods of 1 week each, one in June 1999 and one in December 1999, in emergency departments of five university hospitals and five general hospitals throughout France. All patients aged ≥15 years presenting with medical complaints were included in the study. Trauma patients, those with gynaecological conditions and those with alcohol intoxication or intentional drug poisoning were excluded from the study. Each patient was assessed by two local emergency physicians to determine whether the visit was the result of an ADE. All medical records were subsequently validated by an independent group of medical lecturers in iatrogenic disorders.
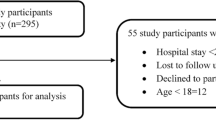
Results: Out of a total of 1937 patients consulting, 1562 were taking at least one drug during the previous week and were included for analysis; 328 (21%; 95% CI 19, 23) of these patients consulted an emergency physician because of an ADE. Patients with ADEs were older than those without (mean age 63.5 vs 54.8 years; p < 0.0001). Furthermore, ADE patients were more likely to have a higher severity presentation than the non-ADE group (p = 0.019). The number of drug exposures was significantly higher in patients with an ADE than in those without (mean number of medications 5.17 vs 3.82; p < 0.0001). On multivariate analysis, only age and the number of medications taken were significantly associated with adverse events. In total, 410 drugs were incriminated in the occurrence of 328 ADEs. The most frequently incriminated drug classes were: (i) psychotropic agents (n = 84; 20.5%); (ii) diuretics (n = 48; 11.7%), anticoagulants (n = 38; 9.3%) and other cardiovascular drugs (n = 63; 15.4%); and (iii) analgesics, including NSAIDs (n = 57; 13.9%). Preventability could be assessed in 280 of the 328 cases. In 106 cases (37.9%), the ADE was judged to be preventable.
Conclusion: ADEs leading to emergency department visits are frequent, and many are preventable, confirming that there is a need to develop prevention strategies.



Similar content being viewed by others
References
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279: 1200–5
Pouyanne P, Haramburu F, Imbs JL, et al. Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. BMJ 2000; 320: 1036
Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med 2000; 109: 87–94
Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003; 289(9): 1107–16
Olivier P, Boulbés O, Tubery M, et al. Assessing the feasibility of using an adverse drug reaction preventability scale in clinical practice: a study in a French emergency department. Drug Saf 2002; 25(14): 1035–44
Winterstein AG, Hatton RC, Gonzalez-Rothi R, et al. Identifying clinically significant preventable adverse drug events through a hospital’s database of adverse drug reaction reports. Am J Health Syst Pharm 2002; 59: 1742–9
Goettler M, Schneeweiss S, Hasford J. Adverse drug reaction monitoring: cost and benefit considerations. Part II: cost and preventability of adverse drug reactions leading to hospital admission. Pharmacoepidemiol Drug Saf 1997; 6Suppl. 3: S79–90
Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. JAMA 1997; 277(4): 307–11
Doucet J, Jego A, Noel D, et al. Preventable and non-preventable risk factors for adverse drug events related to hospital admissions in the elderly: a prospective study. Clin Drug Invest 2002; 22: 385–92
Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med 2003; 348(16): 1556–64
Queneau P, Bergmann JF. Prévention de la iatrogénie évitable: quand la sécurité des malades se conjugue avec les économies de santé. Therapie 2001; 56: 163–8
Begaud B, Evreux JC, Jouglard J, et al. Unexpected or toxic drug reaction assessment (imputation): actualization of the method used in France. Therapie 1985; 40: 115–8
Meyboom RHB, Lindquist M, Egberts ACG. An ABC of drug-related problems. Drug Saf 2000; 22(6): 415–23
Fourestie V, Roussignol E, Elkarrat D, et al. Classification clinique des malades aux urgences: définition et reproductibilité. Rean Urg 1994; 3: 573–8
Hafner JW, Belknap SM, Squillante MD, et al. Adverse drug events in emergency department patients. Ann Emerg Med 2002; 39(3): 258–67
Vital-Durand D. Epidémiologie des effets indésirables des médicaments. In: Guillevin L, editor. Pathologie iatrogène. Paris: Masson, 1993: 1–7
Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients: excess length of stay, extra costs, and attributable mortality. JAMA 1997; 277(4): 301–6
Rothschild JM, Bates DW, Leape LL. Preventable medical injuries in older patients. Arch Intern Med 2000; 160: 2717–28
Carbonin P, Pahor M, Bernabei R, et al. Is age an independent risk factor of adverse drug reactions in hospitalized medical patients? J Am Geriatr Soc 1991; 39(11): 1093–9
Chan M, Nicklason F, Vial JH. Adverse drug events as a cause of hospital admission in the elderly. Intern Med J 2001; 31: 199–205
Faich GA, Knapp D, Dreis M, et al. National adverse drug reaction surveillance: 1985. JAMA 1987; 257: 2068–70
Martin RM, Biswas PN, Freemantle SN, et al. Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: analysis of 48 cohort studies. Br J Clin Pharmacol 1998; 46: 505–11
Ebbesen J, Buajordet I, Erikssen J, et al. Drug-related death in a department of internal medicine. Arch Intern Med 2001; 161: 2317–23
Senst BL, Achusim LE, Genest RP, et al. Practical approach to determining costs and frequency of adverse drug events in a health care network. Am J Health Syst Pharm 2001; 58(12): 1126–32
Lesar TS, Briceland L, Stein DS. Factors related to errors in medication prescribing. JAMA 1997; 277(4): 312–7
Curtis LH, Ostbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164(23): 1621–5
Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA 1999; 282(3): 267–70
Kizer KW. Establishing health care performance standards in an era of consumerism. JAMA 2001; 286(10): 1213–7
Queneau P, Chabot JM, Rajaona H, et al. Iatrogenic disease observed in a hospital setting. II. Analysis of causes and suggestions for novel preventive measures [in French]. Bull Acad Natl Med 1992; 176(5): 651–64
Acknowledgements
Expert Committee Members: Claire Bonithon-Kopp, Gilles Bouvenot, Philippe Casassus, Charles Caulin, Olivier Chassany, Alain Durocher, Jean-Pierre Fauvel, Jacques Kopferschmitt, Dominique Mottier, Jean-Marie Rodrigues, Gérard Duru, André Flory, Laure Papoz, Patrice Pinell, Joseph Lellouch, Pascale Tubert-Bitter, Christine Verdier.
Emergency Department Heads: Bernard Bedock (Annonay), François Bertrand (Nice), Jacques Bouget (Rennes), Françoise Carpentier (Grenoble) Jean-Michel Coulaud (Montfermeil), David Elkharrat (Paris), Thierry Jacquet-Francillon (Bourg-en-Bresse), Jacques Kopferschmitt (Strasbourg), Jacques Nicod (Agen), Eric Roupie (Créteil), Jacques Tourret (Le Puy).
Clinical Research Assistants: Sophie Besson (Grenoble), Alexandre Bucci (Grenoble), Nathalie Dallery (Paris Lariboisière), Charline David (Rennes), Monique Gratteau (Nice), Rachel Portigliati (Annonay), Valérie Raphaël (Montfermeil), Neggar Sedghi (Strasbourg), Albert Trinh-Duc (Agen), Sophie Variclier (Bourg-en-Bresse).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Queneau, P., Bannwarth, B., Carpentier, F. et al. Emergency Department Visits Caused by Adverse Drug Events. Drug-Safety 30, 81–88 (2007). https://doi.org/10.2165/00002018-200730010-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200730010-00008