Article Text
Abstract
Piperacillin-tazobactam is a broad-spectrum antimicrobial agent that is commonly used in clinical practice. The development of delayed drug hypersensitivity reaction (DHR) has been reported in several cases previously. Here we describe an unusual case of non-immediate DHR due to a prolonged course of piperacillin-tazobactam. We report a 22-year-old man who developed fever, eosinophilia, thrombocytopenia and elevated hepatic enzymes following 17 days of piperacillin-tazobactam for methicillin-sensitive Staphylococcus aureus (MSSA) pneumonia. These adverse reactions were reversed immediately after antibiotic cessation. Our case highlights that clinicians should be aware of delayed adverse effects in patients receiving long-term piperacillin-tazobactam treatment.
- drug-related side effects and adverse reactions
- pulmonary medicine
- pharmacy administration
- clinical medicine
- safety
Data availability statement
All data relevant to the study are included in the article.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, an indication of whether changes were made, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- drug-related side effects and adverse reactions
- pulmonary medicine
- pharmacy administration
- clinical medicine
- safety
Background
Piperacillin-tazobactam is a combination formulation of an ureidopenicillin and a beta-lactamase inhibitor with a broad spectrum of antibacterial activity.1 2 It is effective for the treatment of patients with polymicrobial infection caused by many Gram-negative, Gram-positive and beta-lactamase-producing bacteria, such as complicated nosocomial and intra-abdominal infections, and also is regarded as empiric therapy for patients who present with fever and neutropenia.3
As a typical antibiotic, piperacillin-tazobactam is considered safe and well tolerated. However, some adverse reactions have been reported. The most frequent complications include diarrhoea and hepatic dysfunction.4 Eosinophilia with fever and haematological abnormality have been described as severe adverse effects of piperacillin-tazobactam, and are also characteristically regarded as part of the non-immediate drug hypersensitivity reactions (DHRs).5 6 Non-immediate DHRs tend to occur in patients receiving piperacillin-tazobactam therapy over 10 days.7 Here we report an unusual case of a non-immediate DHR reaction to piperacillin-tazobactam.
Case presentation
A previously healthy 22-year-old man presented at our emergency room in January 2020 with high fever (up to 40℃) for a week, accompanied with cough and expectoration. He had 3- year history of smoking without intravenous drug abuse. Physical examination revealed: body temperature 39.5℃; blood pressure 101/54 mmHg and tachypnea of 28 breaths/minute. There were no signs of wounds or skin abscesses. No obvious rhonchi was heard at bilateral lungs and no murmurs were audible in the cardiac valve areas. Initial laboratory tests in the emergency room included: white blood cell count 30.45×109/L, neutrophils 26.95×109/L, platelets 469×109/L, C-reactive protein (CRP) 78 mg/L (normal range 0–8 mg/L) and lactate dehydrogenase (LDH) 657 U/L. Computed tomography (CT) of the chest indicated multiple patchy opacities with cavities. The patient was admitted for further investigation and treatment.
Cardiac ultrasound indicated no signs of bacterial endocarditis. Methicillin-sensitive Staphylococcus aureus (MSSA) was isolated from sputum. Repeated blood cultures were negative. HIV and hepatitis B virus tests were negative. Tests for active and latent tuberculosis, including mycobacterium cultures of sputum samples and the interferon-γ release test for tuberculosis infection, were negative. Chest CT showed multiple dense consolidation with cavities at both side of the lungs and also indicated some cavities with gas-liquid level formation inside pulmonary abscesses (figure 1). A small quantity of pleural effusion presented on the scan. No obvious abnormality was found on abdominal CT. A diagnosis was made of S. aureus pneumonia, complicated by pulmonary abscess. Initially the patient empirically received intravenous vancomycin, 1 g twice daily, and imipenem 1 g every 6 hours. Three days later the patient’s body temperature had markedly decreased. Give the presence of MSSA and abnormality of liver function, antimicrobial therapy was switched to intravenous piperacillin-tazobactam 4.5 g every 8 hours per day.
Computed tomography (CT) images of a 22-year-old patient admitted with Staphylococcus aureus pneumonia in January 2020. CT showed multiple patchy opacities with cavities, some of which had gas-liquid level formation inside in the emergency room (A) and after admission (B). During the initiation of vancomycin and imipenem, slightly absorbed parts of inflammatory lesions were revealed on CT (C). Piperacillin-tazobactam treatment improved the CT findings on day 6 following administration (D). No indication of new infection was found after drug-induced fever. (E). No obvious focus was seen on CT scan 3 months after discharge (F).
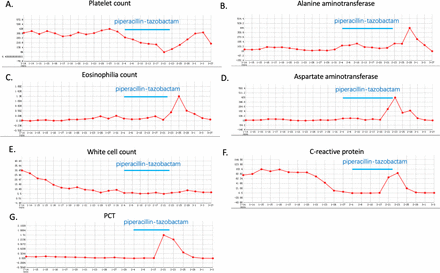
Intravenous piperacillin-tazobactam apparently further improved the patient's symptoms and CT appearance. His body temperature remained normal for 10 days. However, 17 days after continuous treatment with piperacillin-tazobactam, the patient suddenly developed a high fever with a body temperature of 38.5℃ and did not complain of any other symptoms. Detailed physical examination did not reveal any abnormal findings. Blood samples were taken for culture. Repeated chest CT did not show any deterioration or was suggestive of a new infection for the recurrence of fever. Laboratory analysis indicated reduced platelets (66×109/L) compared with the initial level (figure 2). On the following days, the patient also had eosinophilia (1.36×109/L) and liver function abnormality (alanine aminotransferase (ALT) 78 U/L, aspartate aminotransferase (AST) 223 U/L). Potential viral infection of the upper respiratory tract was excluded.
The development of eosinophilia, thrombocytopenia and liver function test abnormality during the course of piperacillin-tazobactam treatment with normal values for white cell count. Blood counts of eosinophils and platelets returned to normal levels after cessation of piperacillin-tazobactam on day 14 following initiation. Liver parameters returned to normal after 1 week following cessation of piperacillin-tazobactam treatment. PCT, procalcitonin.
The piperacillin-tazobactam was suspected to be the most likely cause of the recurrent fever, eosinophilia, thrombocytopenia and hepatic abnormalities, and hence was stopped. The fever quickly resolved within 24 hours following the termination of piperacillin-tazobactam treatment and did not recur afterwards. Platelet and eosinophil counts normalised on day 3 and day 7 after cessation of piperacillin-tazobactam, respectively. The parameters of hepatic function declined nearly to normal levels after a week following piperacillin-tazobactam cessation. The patient did not receive any additional medication (including antibiotic therapy) for the recurrent fever until discharge, and underwent supportive treatment without any complications.
Investigations
This is an uncommon case of adverse drug response caused by piperacillin-tazobactam. Previous reports have revealed several cases of haematological abnormalities that were suspected as probably being piperacillin-tazobactam-induced side effects, including neutropenia, thrombocytopenia or anaemia.8 9 Other reports showed that eosinophilia with systemic symptoms are regarded as kinds of adverse effects caused by the antibiotics.5 10 In our work, we found a special case of delayed thrombocytopenia and eosinophilia that occurred simultaneously after the use of piperacillin-tazobactam for the treatment of S. aureus pneumonia. The patient’s laboratory and clinical factors did not show evidence of latent infection in other locations. The possibile involvement of the upper respiratory tract was excluded.
Treatment
The patient' diagnosis was S. aureus pneumonia, complicated by pulmonary abscesses. The use of intravenous piperacillin-tazobactam apparently improved the patient's symptoms and CT appearance. Although the patient developed fever, eosinophilia, thrombocytopenia and liver damage caused by the treatment, the unusual syndromes gradually disappeared after cessation of piperacillin-tazobactama.
Outcome and follow-up
The fever resolved within 24 hours after stopping piperacillin-tazobactam treatment. The platelet and eosinophil counts both returned to normal after the cessation of piperacillin-tazobactam. The parameters of hepatic function also declined nearly to normal levels following treatment termination.
Discussion
In the present case, the patient started to show the side effects of fever, thrombocytopenia and eosinophilia after 17 days of piperacillin-tazobactam treatment. This is similar to previous reports in which drug induced-fever and associated adverse effects occurred from 11 to 17 days after the initiation of piperacillin-tazobactam administration. We have experienced this several times and have described this as the ‘dangerous period’ for piperacillin-tazobactam-induced fever. Consequently we stopped piperacillin-tazobactam therapy immediately without starting any other antibiotics or medication. Fortunately, this patient's fever disappeared within 24 hours of the piperacillin-tazobactam therapy being discontinued. Although the patient's blood CRP and procalcitonin increased in tandem with the fever, they soon declined on day 3 after the termination of piperacillin-tazobactam therapy. Based on the evidence, we believe there is a correlation between the described side effects and piperacillin-tazobactam therapy.
It has been reported in some cases that piperacillin-tazobactam-induced fever is regarded as a hypersensitivity reaction. Fever, eosinophilia, thrombocytopenia and liver damage are all part of the drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome.5 DRESS syndrome is a life-threatening, rare adverse effect that frequently develops following exposure to antibiotics or antiepileptics, which is characterised by fever, rash, elevated liver enzyme levels, and leukocytosis with eosinophilia.11 The RegiSCAR (European Register of Severe Cutaneous Adverse Reactions) is a scoring system which is most commonly used to categorise cases of DRESS into four groups: no case, possible case, probable case and definite case. Our patient presented several of the described features including fever, eosinophilia and elevated liver enzymes. Typical rash and enlarged lymph nodes were not detected in our patient. Use of the RegiSCAR indicated a possible relationship (score of 3) between our patient’s manifestation of DRESS syndrome and treatment with piperacillin-tazobactam.12
Our patient’s presentation of a fever and an elevated CRP lasting for a transient period evoked a suspicion of latent viral and haematological infection probably after prolonged use of piperacillin-tazobactam. However, no other probable aetiology of eosinophilia could be identified, and the eosinophil abnormality associated with administration of piperacillin-tazobactam resolved soon after the termination of antibiotic therapy. We reasoned that the eosinophilia was probably related to piperacillin-tazobactam as a non-immediate DHR. The mechanism of piperacillin-tazobactam-induced eosinophilia is not entirely understood, but one theory is that activation of interleukin-5 (IL-5) on accumulation of toxic metabolites could cause activation of eosinophils and inflammatory cascades.13 Although there is no accurate diagnostic test or pathognomonic sign, the diagnosis of DRESS syndrome should be considered after exposure to piperacillin-tazobactam for longer than 2 weeks.
Bone marrow suppression is a rare and serious adverse effect of piperacillin-tazobactam, and it has been reported that abnormalities occurred after 2 weeks of prolonged antibiotic use.8 The most common abnormality is neutropenia that is often accompanied with thrombocytopenia. Thrombocytopenia rarely develops alone. Thus, we demonstrate an unusual isolated thrombocytopenia after a long course of piperacillin-tazobactam. To our knowledge, isolated thrombocytopenia is thought likely to be due to an immune-mediated reaction induced by drug administration that occurs suddenly and precipitously. Bose et al described a case of abrupt and severe thrombocytopenia within 2 days of piperacillin-tazobactam administration.14 The patient had a positive test for immunoglobulin G (IgG) antiplatelet antibodies to piperacillin-tazobactam therapy, supporting the speculation of immune-related thrombocytopenia. Alzahrani et al reported two cases of possible immune thrombocytopenia that developed rapidly and severely in association with re-exposure to piperacillin-tazobactam.15 Also the platelet count in peripheral blood improved after the discontinuation of piperacillin-tazobactam and the initiation of corticosteroid therapy, suggesting the possibility of immune-mediated thrombocytopenia.
In our case, the patient developed wild thrombocytopenia without any positive results of auto-immune antibodies or change in complement C3. Since we stopped piperacillin-tazobactam immediately after the side effect occurred, the thrombocytopenia quickly resolved without corticosteroid therapy. It has been suggested that this later-onset thrombocytopenia is dependent on a delayed-type hypersensitivity. Among antibiotics, the beta-lactam antibiotics are a common cause of immune thrombocytopenia, while a beta-lactam-beta-lactamase inhibitor combination, such as piperacillin-tazobactam, has rarely been reported to induce immune-mediated thrombocytopenia. The aetiology of drug-induced immune thrombocytopenia is complex and the mechanism of piperacillin-related thrombocytopenia is possibly associated with hapten-induced antibodies. Some studies have suggested that small molecules like drugs might induce an immune response when linked to macromolecules such as proteins. The combination acts as a hapten and triggers a humoral immune response. On re-exposure to the drug, this leads to platelet destruction and this mechanism may explain the haemolytic anaemia.16 Another report revealed that piperacillin can induce hapten-specific antibodies that are reactive with piperacillin-coated blood cells.17 This similar mechanism may account for the thrombocytopenia that is rarely seen in patients after treatment with piperacillin, but this still needs further confirmation experimentally. An explanation whereby the later-onset thrombocytopenia caused by piperacillin-tazobactam likely occurs as a result of direct toxicity of myeloid precursors should not be excluded.
Moreover, our patient also manifested liver dysfunction, having previously had normal liver function, while the haematological abnormality appeared after the onset of drug-induced fever, and the hepatic value returned to the normal level 1 week after drug cessation. Although hepatic dysfunction has been reported previously in patients on piperacillin-tazobactam therapy, there is no apparent relationship with mortality or progression. Saloojee et al showed that among 225 critically ill patients enrolled in a retrospective study, piperacillin-tazobactam was found to be associated with hepatic dysfunction18 . McDonald et al showed there were no significant differences between the high-dose and licensed dose of piperacillin-tazobactam therapy in terms of hepatotoxicity.19 Another report indicates that the beta-lactamantibiotics may induce leucopenia in sever liver dysfunction.20 In the present case, there was no other explanation for this complication except for the exposure to piperacillin-tazobactam as there was no evidence of sepsis, or other responsible drugs. We speculated that the hepatic dysfunction was associated with antibiotic use.
In conclusion, this is the first description of an unusual case of fever, eosinophilia, thrombocytopenia and liver damage induced by piperacillin-tazobactam. The antibiotic-induced fever may precede bone marrow suppression. Eosinophilia is a rare adverse effect of piperacillin-tazobactam and caution is needed regarding the DRESS syndrome in patients with long duration of piperacillin-tazobactam treatment.
Learning points
This is an unusual case of adverse effects induced by long-term use of piperacillin-tazobactam that has not been observed previously for methicillin-sensitive Staphylococcus aureus.
Eosinophilia is rarely seen following piperacillin-tazobactam treatment, especially accompanying other adverse effects simultaneously.
Clinicians should be aware of possible adverse effects in patients receiving long-term antibiotic treatment.
Data availability statement
All data relevant to the study are included in the article.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors JL: overall manuscript writing and interpretation. GW and FZ: interpretation of results. XS: manuscript modification and submission.
Funding This study was funded by National Natural Science Foundation of China (82070011, 81670073), Jiangsu Commission of Health (K2019004) and "333 project" of Jiangsu Province (BRA2019339).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.